Chapter 15
Chapter 15 
REFLEXES
If you look at every textbook of neurophysiology and neurology in the library, you will not find many that give a definition of the term reflex. It is difficult to define the term in a way that includes everything we call a reflex, yet says anything that allows one to decide if any particular event is a reflex. We will follow suit in a sense and define a reflex as "a relatively stereotyped movement or response elicited by a stimulus applied to the periphery, transmitted to the central nervous system and then transmitted back out to the periphery." Most reflexes involve activities that are nearly the same each time they are repeated, but no activity of an organism is fixed and independent of either the state or the history of the organism. Most reflexes involve the simplest of neural circuits, some only two or a few neurons; but many, like the scratch-reflex in a dog, are so complicated that their organization remains a mystery. Most reflexes are "involuntary" in the sense that they occur without the person willing them to do so, but all of them can be brought under "voluntary" control. Some reflexes serve protective functions, like the eyeblink reflex. Some reflexes act as control systems to maintain homeostasis in some bodily systems.
There are a number of ways of classifying reflexes. One is in terms of the systems that receive the stimulus and give the response. There are viscerovisceral reflexes, for example the decrease in heart rate that follows distention of the carotid sinus; viscerosomatic reflexes, like the abdominal cramping that accompanies rupture of the appendix; somatovisceral reflexes, such as the vasoconstriction that results from cooling the skin; and somatosomatic reflexes, like the knee jerk that follows tapping the patellar tendon. Reflexes can also be classified in terms of the number of neurons or synapses between the primary afferent neuron and the motor neuron. We distinguish two types, the monosynaptic reflex and the much more common multisynaptic or polysynaptic reflex. The term multisynaptic implies that more than one synapse is involved, whereas polysynaptic usually implies that the pathway is of variable length, some parts disynaptic, some trisynaptic, etc.
| The tendon jerk reflex |
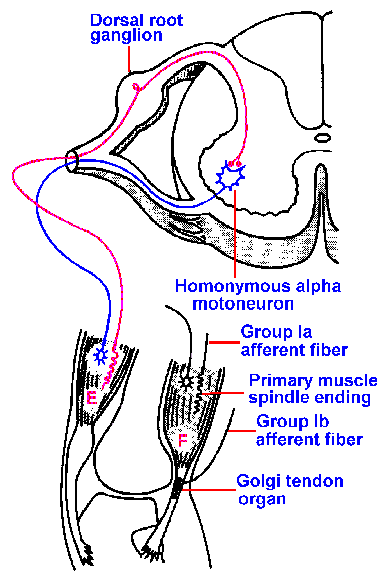 |
Fig. 15-1. The tendon jerk reflex. A circuit diagram of the elements of the tendon jerk reflex: the muscle spinlde, group Ia afferent fiber, alpha-motoneuron, and extrafusal muscle fiber. Note that this is a monsynaptic reflex. F and E indicate flexor and extensor muscles. (Schadé JP, Ford DH: Basic Neurology. Amsterdam, Elsevier, 1965) |
The simplest reflex is the monosynaptic reflex or the two-neuron reflex, an example of which is the tendon jerk reflex or tendon tap reflex, sometimes called the myotatic reflex. This is the reflex that is elicited by tapping the tendon just below the patella. The tap, applied to the tendons of the quadriceps muscles, stretches the muscles and their muscle spindles. A brisk tap excites the group Ia afferent fibers, because of their velocity sensitivity, ultimately causing the muscle to contract. The neural circuit for this reflex is shown in Figure 15-1(1). The group Ia afferent neuron enters the spinal cord through the dorsal root, penetrates into the ventral horn, and then synapses on an alpha-motoneuron. (This is the only synapse in the pathway within the spinal cord, thus the reflex is monosynaptic.) The axon of the alpha-motoneuron then exits the spinal cord through the ventral root and innervates the extrafusal fibers of the muscle from which the group Ia afferent fiber originated, i.e., the homonymous muscle. Note that the drawing shows only one neuron of each type, afferent and efferent, but that one represents many. For example, the cat soleus muscle contains about 50 group Ia afferent fibers and each soleus alpha-motoneuron appears to have a synaptic connection with each one of those 50 group Ia afferent fibers. A brief tap on the tendon will therefore activate many of the group Ia afferent fibers, producing contraction of many of the soleus muscle fibers.
Tapping the tendon of the rectus femoris muscle of the quadriceps group produces a brief stretch of that muscle that acts as a powerful stimulus for the group Ia afferent fibers of the muscle, causing them to give a brief, synchronous discharge. Each discharge, after propagating down the group Ia axon to its termination, produces an EPSP in the rectus alpha-motoneurons. Because there are many EPSPs from many group Ia afferent fibers occurring nearly simultaneously in some alpha-motoneurons, the membrane potentials reach critical firing level (by spatial summation) with hypopolarization to spare, and the motoneurons discharge action potentials. The action potentials travel out by way of the ventral root to the muscle and, because the neuromuscular junction is an obligatory synapse, the muscle contracts. The contraction in turn causes the spindle to be unloaded or shortened passively, its equatorial region to relax, the group Ia afferent fiber to turn off, and the muscle to relax. This is the tendon jerk reflex.
Many of the homonymous alpha-motoneurons are not discharged by the Ia afferent fiber input, but have EPSPs evoked in them that do not achieve the critical firing level. The excitability of the motoneuron is therefore increased. This group of excited neurons is called the subliminal fringe. The presence of the subliminal fringe accounts for enhancement of the reflex response under certain circumstances, for example with the Jendrassik maneuver. In the Jendrassik maneuver, the fingers of the two hands are locked together and one hand pulls against the other while the tendon tap reflex is evoked. The reflex evoked is stronger than in the absence of the maneuver. (Interestingly, mental arithmetic and a number of other activities will do the same thing!) During the Jendrassik maneuver activity, originating perhaps in the cervical enlargement of the spinal cord or some other rostral center, descends the spinal cord to excite alpha-motoneurons. This activity by itself does not cause the alpha-motoneurons to discharge or the muscle to contract, but when added to the subthreshold excitation of the subliminal fringe caused by the tap-induced muscle stretch, it causes the neurons in the subliminal fringe to discharge. The reflex contraction will therefore be larger than normal. There may also be some influence of increased gamma-motoneuron activity, increasing the sensitivity of the primary spindle endings, but this influence should be small because the stimulus for the reflex is very brief.
The value of the stretch reflex mechanism may not be clear at first, but some reflection may clarify its role in motor control. It is unlikely that muscles undergo such rapid stretches very often, with the possible exception of when a person jumps off a wall or jumps up and down on a pogo stick. However, in these instances, the rapid stretch of the rectus femoris that occurs when the feet or the pogo stick contract the ground causes a reflex contraction that helps prevent the gluteus from being overly bruised.
Usually, the postural muscles experience relatively slow, sustained stretches and the anti-gravity muscles, of which the quadriceps is an example, are pulled upon by gravitational forces. This steady force sets up a sustained discharge in each group Ia afferent fiber, but the discharges in different fibers are not synchronized as they are when the tendon is tapped. In addition, longer, larger stretches are able to excite secondary muscle spindle receptors which also have connections with homonymous alpha-motoneurons, di- and trisynaptic ones. These longer, larger stretches therefore activate the alpha-motoneurons by both monosynaptic and polysynaptic reflex pathways. The resulting reflex contraction of the muscle is called the stretch reflex. The polysynaptic effects are not seen in the tendon tap reflex for two reasons: (1) the brief stretch does not excite secondary spindle receptors and (2) the brief input over the polysynaptic pathways arrives after the monosynaptic input and finds the alpha-motoneurons in their refractory periods and therefore cannot cause them to discharge again.
In controlling posture, the asynchronous discharge in mono- and polysynaptic pathways induced by gravitational forces on muscles sums in the alpha-motoneuron with other activity from within the CNS to produce a contraction that just balances the gravitational force. If an additional force is applied, stretching the muscle, additional tension is developed by the stretch reflex to counteract that force. In this way, the stretch reflex serves as a mechanism for maintaining an upright body orientation under a variety of load conditions; the mechanism is automatic ("unconscious") and fast (19-24 msec for the quadriceps in man).
In addition to the monosynaptic connections of the group Ia afferent fibers with the homonymous alpha-motoneurons (e.g., rectus femoris Ia with rectus alpha-motoneuron), there are also monosynaptic connections with synergistic alpha-motoneurons, those innervating muscles that act in the same way at the same joint, but the effects are not as strong in the synergists as they are in the homonymous alpha-motoneurons. Thus, the rectus group Ia afferent fibers also excite the vastus alpha-motoneurons, though not as strongly as they do the rectus alpha-motoneurons. Fewer of the synergistic alpha-motoneurons actually discharge, and the subliminal fringe is larger than for homonymous alpha-motoneurons.
Most skeletal muscles exhibit a tendon tap reflex, but the reflex is strongest in the antigravity or physiological extensor muscles. This makes some sense in light of the discussion of the last paragraph. Note that physiological extensors are not necessarily anatomical extensors. The biceps brachii are a case in point; they are anatomical flexors of the elbow but they are physiological extensors, moving the forearm against gravity.
It is clinically important to note that these reflexes involve only one or two segments of the spinal cord. In fact, the spinal cord can be cut above and below these segments, and the reflexes will still occur. For this reason, testing such reflexes cannot be used as an indicator of the condition of the brain or even other segments of the spinal cord.
| Reciprocal innervation |
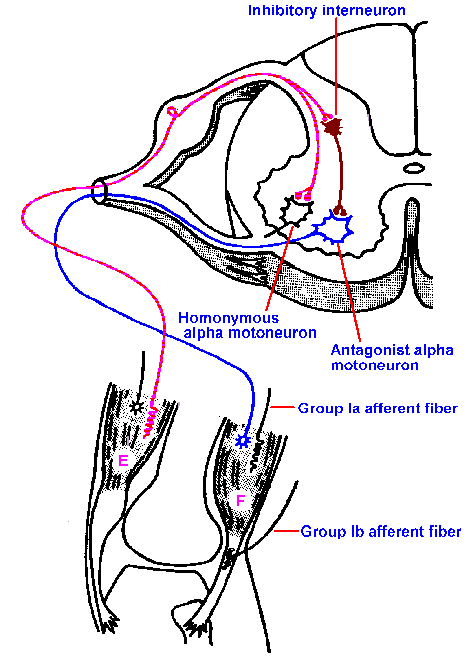 |
Fig. 15-2. Reciprocal innervation. A circuit diagram showing the collateral of the group Ia afferent fiber synapsing on an inhibitory interneuron that synapses on the alpha-motoneruon of the antagonist muscle. F and E indicate flexor and extensor muscles. (Schadé JP, Ford DH: Basic Neurology. Amsterdam, Elsevier, 1965) |
When the extensor muscle contracts during such a reflex, there is usually a relaxation of its antagonists, the flexor muscles crossing the same joint. If this did not occur, the reflex movement could be resisted and diminished by the force of the antagonist muscle(2). The neural mechanism underlying this relaxation of the antagonist muscles is shown in Figure 15-2. The group Ia afferent fiber, after entering the spinal cord, gives off a collateral branch that synapses on an interneuron. This interneuron, in turn, synapses on the antagonist alpha-motoneuron, in the case of our previous example, a hamstring motoneuron. Its effect on the hamstring alpha-motoneuron is inhibitory, i.e., it causes an IPSP in the alpha-motoneuron that is ultimately manifested as a relaxation of the muscle. This is reciprocal inhibition. Notice that this is a polysynaptic reflex pathway.
It is a general principle that anything that has an excitatory (or inhibitory) influence on an alpha-motoneuron also inhibits (or excites) the alpha-motoneurons of its antagonist muscle. This is the principle of reciprocal innervation. Thus, for example, excitation of the hamstring alpha-motoneurons by group Ia afferent fibers is accompanied by inhibition of quadriceps alpha-motoneurons. Reciprocal inhibition is a specific example of the more general principle of reciprocal innervation.
| Dale's principle |
At this point, it is appropriate to bring up the reason for interneurons in pathways such as that in Figure 15-2. Dale's principle (formulated by Sir Henry Dale) states that a single neuron synthesizes only one transmitter substance. Therefore according to the principle, if a neuron secretes acetylcholine at one of its terminals, it secretes acetylcholine at all of its terminals. Sir John Eccles has extended this notion to say that the effect of the transmitter released by a single fiber is the same at all its terminals. This would imply that the net result of activity in all group Ia afferent fibers is excitation and only excitation. Thus, if it is necessary to have inhibition in a pathway involving group Ia afferent fibers, then an inhibitory interneuron must be interposed.
Some neurons make, or at least contain, more than one of the putative transmitter substances discussed in Chapter 13. However, it is not known whether all of the substances made by a neuron are actually used as transmitter substances by that neuron. For example, some neurons contain both GABA and somatostatin or GABA and some neuropeptide. Many peptides are thought to be neuromodulators rather than traditional transmitter substances. Therefore, it is not clear that this is a violation of Dale's principle. On the other hand, the idea that the effect of a transmitter substance is everywhere the same is not tenable. It is now known that certain neurons in Aplysia californica, the sea slug, secrete acetylcholine at synapses with two different postsynaptic cells, and, in one cell, the transmitter substance evokes an EPSP and, in the other, an IPSP. Thus, the action of the transmitter substance on a neuron depends upon that neuron and the receptors or channels it possesses not on the transmitter substance or the neuron that released it. Whether this behavior is also a characteristic of mammalian neurons is not yet certain. Nevertheless, the usual approach in neurophysiology has been to interpose an interneuron whenever the sign of the effect changes, whether there is direct evidence of an interneuron or not.
| The flexion reflex |
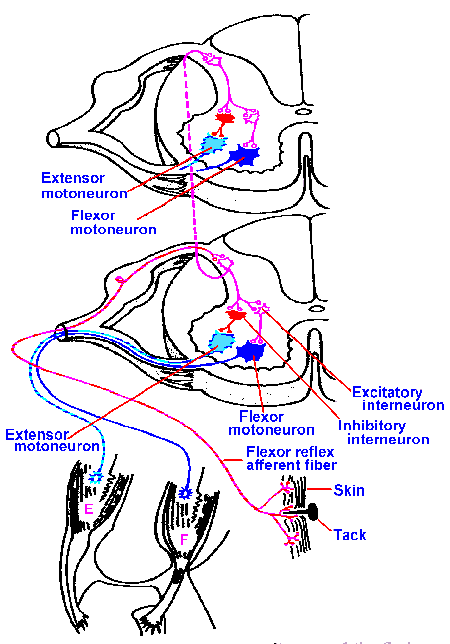 |
Fig. 15-3. Flexion reflex. A circuit diagram of the flexion reflex showing afferent fibers from skin, interneurons, and flexor alpha-motoneurons in two spinal cord segments. Note that some interneurons are intersegmental. F and E indicate flexor and extensor muscles. (Schadé JP, Ford DH: Basic Neurology. Amsterdam, Elsevier, 1965) |
If you have ever touched a hot object or stepped on a sharp object and withdrawn your hand or foot, you have experienced a flexion reflex, a nocifensive reflex, or a withdrawal reflex, all terms describing the same event. The protective result of this reflex is obvious; it quickly removes the part of the body from the vicinity of the offending object by contracting the appropriate muscles, usually flexors, and relaxing extensor muscles (again, reciprocal innervation). The vigor of the reflex depends upon the strength of the stimulus. A weak pinch produces flexion of the foot; a slightly stronger one, flexion of both the foot and the leg; and a very strong one, flexion of foot, leg, and even hip. This spread of the reflex with stronger stimulation is called irradiation. The exact nature of the limb movement and the final position of the limb vary depending upon the site of stimulation. This phenomenon is often called local sign. Because of local sign, the withdrawal of the limb from damaging stimuli is usually appropriate in both magnitude and direction.
The pathways for the flexion reflex are illustrated in Figure 15-3. The afferent limb (the part going to the spinal cord) of this reflex consists of nociceptors with A or C fibers and fibers of groups II, III, and IV of muscle. These are sometimes referred to collectively as the flexor reflex afferent fibers. They enter the spinal cord and synapse on interneurons, whose axons distribute to other interneurons that affect alpha-motoneurons within the same and in different segments of the spinal cord. Notice that this is a polysynaptic reflex. Activity in the nociceptive afferent fibers in the common peroneal nerve serving the first and second toe leads to discharge of the peroneus alpha-motoneurons, which, in turn, leads to dorsiflexion of the foot. If the nociceptive activity is strong enough, it is able to activate other peroneus alpha-motoneurons, further increasing the flexion of the foot, and also to bring in alpha-motoneurons of synergistic muscles of the foot, as well as other related muscle groups, for example the hamstrings, to lift the leg. This may involve transmission to spinal segments other than the segment of entry.
| The crossed-extension reflex |
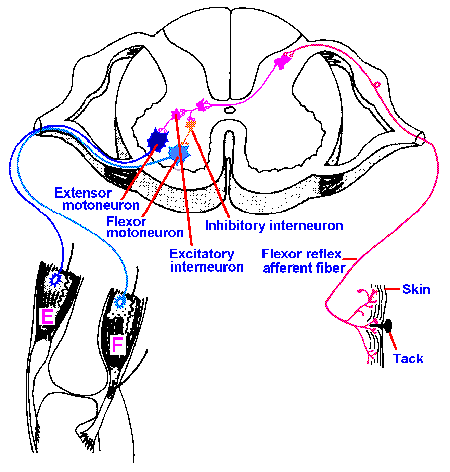 |
Fig. 15-4. Crossed-extension reflex. Circuit diagram showing branching of the flexor reflex afferent fibers and their termination on an interneuron that decussates and terminates on contralateral extensor alpha-motoneurons. The circuit for the contralateral reciprocal inhibition of flexor alpha-motoneurons is also shown. F and E indicate flexor and extensor muscles. (Schadé JP, Ford DH: Basic Neurology. Amsterdam, Elsevier, 1965) |
If protection of the limb requires it to be elevated, then the rest of the body is imperiled by removal of the support the limb normally provided, unless some compensation is made. The reflex contraction of flexor muscles on one side of the body is always accompanied by contraction of the extensor muscles of the contralateral limb. This gives increased antigravity support on the contralateral side to hold the body upright and is called the crossed-extension reflex. The circuit for this reflex is illustrated in Figure 15-4. The flexor reflex afferent fibers also synapse on interneurons that decussate (cross the midline) and terminate on contralateral extensor alpha-motoneurons. This pathway is polysynaptic and purely excitatory. In addition, there is the usual reciprocal inhibitory effect on the contralateral flexor alpha-motoneurons.
| Autogenic inhibition |
Activity in the group Ib afferent fibers, associated with Golgi tendon organs, inhibits the homonymous alpha-motoneurons. The inhibition is disynaptic involving one interneuron and two synapses between the afferent fiber and the motoneuron. The effect is called autogenic inhibition (sometimes written autogenetic inhibition). Like the group Ia excitation, group Ib inhibition is exerted not only on the homonymous alpha-motoneurons, but also on the alpha-motoneurons of synergistic muscles. Reciprocal innervation is also present here, with group Ib activity exciting the alpha-motoneurons of the antagonist muscles.
It was originally thought that this inhibition served only to protect the muscle from being injured when contracting against too heavy a load. This concept came from two sources: (1) the supposed high threshold of group Ib fibers to tension (not true for developed tension) and (2) the existence of a clasp-knife reflex. The clasp-knife reflex exists only in certain pathological conditions, e.g., "upper motoneuron" disease. Under these conditions, there is an extensor spasticity that resists any attempt to flex the limbs, especially the arms. If the arm is gradually, forcibly flexed by someone other than the patient, a point will be reached when the resistance to flexion suddenly melts away, and the limb collapses easily into full flexion, reminiscent of the action of a pocket or clasp-knife. This is the clasp-knife reflex or the lengthening reaction. It was thought formerly to be mediated by autogenic inhibition and to occur near the threshold for group Ib activation by increased muscle tension. Now it is thought that it occurs at the point where autogenic inhibition is great enough to overcome the stretch reflex excitation, that is, when the membrane potential of the extensor motoneuron falls below the critical firing level and the muscle relaxes. Actually, Golgi tendon organ activity cannot entirely account for the clasp-knife reflex. As the spastic muscle is lengthened, the group Ia discharge continually increases and, because the tension is increasing, the group Ib discharge also increases. The result, at the alpha-motoneuron, is excitation from the group Ia fiber input and inhibition from the group Ib fiber input, the inhibition finally taking dominance and silencing the alpha-motoneuron. The muscle relaxes. This accounts for the relaxation of the spastic muscle, but it does not account for the failure of its immediate return. When the muscle relaxes, the tension falls, and the group Ib fiber discharge ceases. This should decrease the autogenic inhibition and allow group Ia fiber input to reestablish the spasticity, but the muscle stays relaxed until the joint is extended again, whereupon the spasticity reappears. Recent evidence suggests that group Ia fibers from muscle spindles also contribute to autogenic inhibition (Fetz EE, Jankowska E, Johannisson T, Lipski J: J Physiol (Lond) 293:173-195, 1979). Because the muscle does not shorten, but lengthens when the spasticity disappears, the group Ia fibers will discharge even more briskly, increasing both excitation and autogenic inhibition of the alpha-motoneurons. Apparently, the inhibition predominates.
In the normal person, it is certain that the Golgi tendon organ contributes to control of muscle activity over the whole range of movement, not just at its extremes. There is no reflex movement associated with stimulation of Golgi tendon organs in isolation, so that nothing can be said with certainty about their reflex function. Perhaps they do serve a protective function, but they may also be supplying tension information for complicated tension-maintaining reflexes or supplying inhibition at the appropriate moment to switch from flexion to extension movements in walking or running.
They may also play a role in increasing muscle force during fatigue. Thus, during fatigue the muscle produces less force, which reduces Golgi tendon organ activity. This leads to decreased inhibition and, therefore, increased activity of the homonymous -motoneurons. The increased motoneuron activity will lead to greater force of contraction. The problem with this scenario is that the resulting increased force should lead to increased Golgi tendon organ activity and, so, decreased force. How this complicated system is working is yet to be determined.
| Other reflexes |
There are a number of other reflexes commonly tested in the clinic, whose mechanism we do not understand thoroughly. The extensor thrust reflex involves extension of the lower limb when a tactile stimulus is applied to the plantar surface of the foot. This may play some role in walking or standing by maintaining contact with the surface. The Babinski sign is a reflex that is pathological in adults but normal in infants. Scraping the sole of the foot of a normal adult with a tongue depressor results in plantar flexion of the toes. In the infant and the spinal adult (with a spinal cord transection), the same stimulus leads to dorsiflexion of the toes. This pathological change (in adults) is called Babinski's sign, and, as we shall see, it is usually taken as a sign of pyramidal tract damage.
There are a great many other reflexes that could be mentioned here. We have already discussed reflex control of pupil diameter and lens shape in the eye and auditory sensitivity by the middle ear muscles and olivocochlear bundle. In addition, there are stretch receptors in the lungs, probably in the bronchi and bronchioles, that are activated by lung inflation. The activity of these receptors enters the medulla by way of the vagus nerve (Xth cranial nerve) to produce inhibition of inspiratory neurons. This effect, called the Hering-Breuer reflex, has the effect of terminating inspiration when a certain level of inflation has been attained. It is mediated through the nucleus solitarius and the reticular formation.
There are other respiratory reflexes as well. For example, the stretch reflex and autogenic inhibition play a major role in control of the intercostal muscles during inspiration. The gamma loop appears to provide an important facilitation of discharges in -motoneurons to the external intercostals in particular. A most important reflex pathway regulates the concentrations of oxygen and carbon dioxide in arterial blood by adjusting respiratory parameters. Chemoreceptors in the carotid and aortic bodies increase their discharge rates in response to a decrease in arterial oxygen concentration or an increase in arterial carbon dioxide concentration (or, alternatively, a decrease in pH). Sensory fibers from these receptors enter the brain stem through the glossopharyngeal nerve (IXth cranial nerve), where they make connections with cells whose activity results in an increase in respiratory volume and rate, with certain changes in peripheral vasculature, to elevate blood oxygen or decrease blood carbon dioxide levels. In addition, there are chemoreceptors in the CNS that are excited by elevation of the carbon dioxide of the cerebrospinal fluid or a decrease in its pH. Their activity results in the same changes in respiration, apparently without much change in the cardiovascular system.
There are also reflexes involving the heart. Baroreceptors (pressure receptors) in the carotid sinus, in the aortic arch, and perhaps elsewhere, increase their discharge rates in response to elevated blood pressure. This increase in activity leads reflexly to a decrease in sympathetic discharge, resulting in peripheral vasodilatation and in an increase in parasympathetic discharge primarily through the vagus nerve, resulting in decreased heart rate. The net result is a decrease in blood pressure. Stimulation of high threshold (small diameter) primary afferent fibers in cutaneous and muscle nerves also leads to a reflex increase in blood pressure.
Vomiting is the result of a reflex arc involving receptors in the region of the pharynx, supplied by the IXth and Xth cranial nerves and also receptors in the medulla. The medullary receptors, located in a region with high permeability of the blood brain barrier, are sensitive to chemical agents in the blood. The efferent limb of the reflex involves somatic innervation of the abdominal muscles and autonomic innervation of the stomach (smooth) muscles and the cardiac sphincter. During vomiting, the cardiac sphincter relaxes, while the abdominal and stomach muscles contract, expelling the stomach contents through the esophagus.
Swallowing is also a reflex activity. The reflex is triggered by tactile stimulation of the mucosa of the palate, pharynx, and epiglottis. Receptors in these regions, perhaps free nerve endings, send their activity through the IXth cranial nerve, the superior laryngeal branch of the Xth cranial nerve, and perhaps also the Vth cranial nerve. The reflex pathway involves synapses in the nucleus solitarius and efferent fibers to the tongue in the hypoglossal nerve (XIIth cranial nerve) and to the palate, pharyngeal, and esophageal musculature through the IX, X and XIth cranial nerves from the nucleus ambiguous.
Temperature regulation is a complicated reflex activity, involving both peripheral thermoreceptors and central thermoreceptors located in the anterior hypothalamus and perhaps elsewhere. The reflex pathways are not well understood, but they involve hypothalamic activation of the autonomic system, resulting in peripheral vasoconstriction, piloerection, and shivering, if heat needs to be generated or conserved, and in sweating, increased respiration, and peripheral vasodilatation, if heat needs to be lost in maintaining body core temperature at around 37 °C.
Micturition is a reflex activity that depends upon stretch receptors located in series with smooth muscle cells of the bladder wall. This mode of attachment allows the receptors to discharge both when the muscles are stretched and when they contract. Fibers from these receptors are carried in the pelvic nerves to the S2-S4 spinal cord segments. Somatic afferent fibers to these same segments play a role in the reflex as well. Bladder emptying is produced by contraction of the detrusor muscle of the bladder and also muscles of the abdominal wall, with relaxation of the internal (smooth muscle) and external (striated muscle) sphincters. Reflex emptying can be accomplished with an isolated sacral spinal cord, provided that the sacral roots, especially ventral root S3, are intact, but usually the reflex is under strong control from supraspinal centers.
The reflex emptying of the colon, i.e., defecation, is accomplished in a way similar to micturition. Here, the receptors are located in series with smooth muscle of the rectal wall. Tactile receptors in the skin and anus also facilitate defecation, as does the simultaneous occurrence of a micturition reflex. Emptying is under parasympathetic control from the S2-S4 spinal cord segments, producing contraction of the smooth muscle lining the rectum and relaxation of the sphincters. Evacuation is also assisted by contraction of the diaphragm and abdominal muscles with the glottis closed (Valsalva's maneuver), increasing the pressure in the abdominal cavity.
There are also sexual reflexes whose organization is partially understood. The afferent fibers for penile erection and ejaculation reflexes arise from the genitalia and course through branches of the pudendal nerve, again, to spinal cord segments S2-S4, although many other stimuli can elicit sexual responses under appropriate circumstances. Many of these other stimuli must act through higher centers. Both somatic and autonomic efferent fibers are involved in producing erection, with parasympathetic fibers producing vascular engorgement and somatic motoneurons to the bulbocavernosus and ischiocavernosus muscles producing contraction that compresses the venous outflow from erectile tissues. Sympathetic fibers also play a poorly understood role; bilateral sympathectomy reduces or eliminates both erections and ejaculations. Sympathetic, parasympathetic and somatic motor activity are all involved in producing ejaculation.
These are a few of the many reflexes mediated by the nervous system. Some are mediated by the spinal cord and can occur when the spinal cord is disconnected from the brain, but some require intact connections with the brain stem or diencephalon for their operation. All, however, are under the influence of cortical and subcortical structures in their normal operation.
| The function and utility of reflexes |
Many of the reflexes are artifacts of the way we stimulate the body (although many, like the eye blink reflex, are essential to protect body parts from injury and many play specific regulatory roles). However, they tell us two useful things: (1) how the nerve circuits of the spinal cord or brain stem are put together and (2) the condition of various parts of these circuits. The first piece of information is useful to the neurophysiologist, who ultimately wants to know how the nervous system works. However, we will see in the next chapter that many reflexes are suppressed or modified during phases of the stepping cycle in order to prevent muscles from contracting at the wrong times. The state of a reflex circuit may be highly variable depending upon the condition of a person, e.g., awake, asleep, comatose; upon what he is doing, e.g., walking, running, thinking; upon his position in space or posture; and upon other factors. Therefore, the reflex pattern at any one time may not be a good indicator of the neural circuits available in the nervous system or of how they behave under a variety of conditions.
The second piece of information is useful to the clinician, who wants to know what is wrong with a patient. An example may help to show how the reflexes are used clinically. A patient complains of paresis or weakness of the right leg. There are three possible causes for this paresis: (1) he is suffering from disease of descending tracts having to do with leg function; (2) he is suffering from disease of the -motoneurons themselves; or (3) something is wrong with the muscle. A normal tendon tap reflex rules out the last two alternatives, because the -motoneurons and muscle must be functional to give a normal reflex.
| Reflex pathways as control systems |
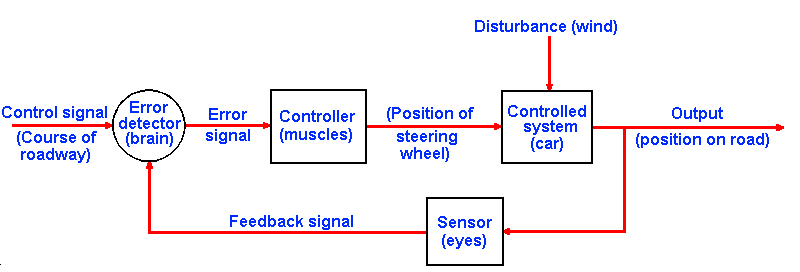
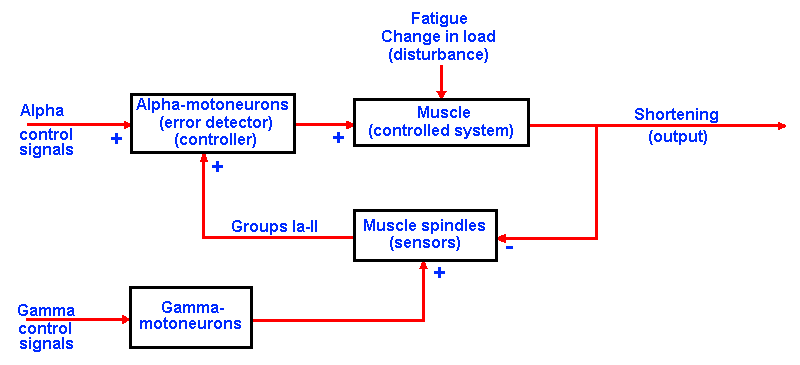
One way to think of the function of reflexes is as control systems(3), systems that regulate the various parameters of muscle contraction. The essential feature of this type of system is the more-or-less continuous flow of information from the element controlled back to the device that controls it. This is called feedback. An everyday example of feedback is the continuous information your eyes give you about the course your automobile is taking. Without visual information, you are likely to run off the road before you can detect errors in your steering. With visual feedback, small corrections can be made in your steering before you leave the road.