 Chapter 16
Chapter 16 
INITIATION AND CONTROL OF MOVEMENT
Control over movement is exerted by all parts of the nervous system, not just those identified as "motor" in textbooks. The participation of alpha-motoneurons in motor control is obvious; without them there can be no movement! Considering our knowledge of reflex pathways, the role of the primary afferent neurons, certain interneurons, and the gamma-motoneurons seem fairly evident. Most of the nerve fibers that innervate a muscle are there to sense and control the length and tension of the muscle, not to make it contract. This is clearly seen in Table 16-1. In the cat soleus muscle, there are about 50 group Ia fibers, 50 group II fibers, 40 group Ib fibers, and 100 gamma-motoneurons compared with only 150 alpha-motoneurons. This means the muscle has 240 nerve fibers for control and only 150 for contraction. Clearly the bias is in favor of control.
Innervation of the Cat's Soleus Muscle
| Afferent fibers | 50 group Ia fibers - 50 muscle spindle primary endings |
| 40 group Ib fibers - 45 Golgi tendon organs | |
| 50 group II fibers - 50 muscle spindle secondary endings | |
| Efferent fibers | 100 gamma-motoneurons - 300 intrafusal muscle fibers in 50 muscle spindles |
| 150 alpha-motoneurons - 25,000 extrafusal muscle fibers |
At the spinal cord segmental level, a considerable organization of movement is already apparent as indicated by reflex patterns in spinal animals. Reciprocal innervation is the sort of mechanism of coordination one might expect to find in the spinal cord to "allow" movement to occur, but other reflex patterns, such as the compensation contributed by the crossed-extension pathway, may not be entirely expected. In addition, in the spinal cord, there are even higher levels of coordination. The scratch reflex in animals, for example, is mediated entirely at the spinal cord level, and patterns of movement reminiscent of walking, i.e., a series of alternating flexions and extensions of the limbs, can be seen in the spinal human or animal. It has been shown that the stepping cycle in experimental animals depends upon rhythmic activity that is generated in the spinal cord itself and not by primary afferent fibers or descending influences. This is not to say that walking is entirely a spinal cord event, but many of the neural circuits for the behavior are there. There is more to walking, however, than just the generation of the stepping cycle. It is also necessary to coordinate the movements of upper and lower limbs during walking or running and, to a certain extent, this also appears to be mediated at the spinal cord level. The stepping must be performed in relationship to the environment so that the foot is put on level ground and not in a hole, so that walking is in relationship to the intended course, and so that moving obstacles can be avoided. These adjustments and corrections are the province of supraspinal structures. We will have more to say about this subject later.
| Spinal cord transection |
A view of the net effect of supraspinal pathways (pathways descending from structures above the spinal cord) upon the spinal cord circuits can be seen in experimental cord transections or in cases of accidental cord transection in humans. With modern car and especially motorcycle traffic, this is not a terribly rare event in humans, and if it occurs below the level of the spinal roots containing fibers of the phrenic nerve (C3-C5), the chances of survival without lifesupport systems are good because the diaphragm still functions. Immediately after transection of the spinal cord, all movement as well as all sensation and all reflexes are lost in the parts of the body innervated by the segments below the cut. This temporary condition is called spinal shock. The duration of spinal shock is variable, though usually of the order of several weeks in humans, less long in other species. Spinal shock is understandable in terms of the excitability of central neurons, among them the alpha-motoneurons, which is determined by the sum total of the excitatory and inhibitory synaptic inputs to them (see Chapter 13). Apparently, the net effect of removing all supraspinal influences on motoneurons is the elimination of a significant excitation. Under these conditions, even the normally powerful group Ia afferent fibers are without their usual effect as indicated by the resulting areflexia, i.e., the absence of reflexes. This points out again the importance of summation in setting the excitability levels and firing patterns of neurons.
An indicator of the onset of the period of recovery from spinal shock is the appearance of flexion reflexes, which are mediated by polysynaptic pathways. One of the earliest to appear is the Babinski sign, which is a symptom associated with pyramidal tract disorder. Over time, the flexion reflexes become stronger and usually become hyperactive to the point that a small stimulus sets off a generalized flexion reflex. During this period of increased flexor reflex activity, the stretch reflexes make their appearance and grow in excitability until they are exaggerated. With the hyper-reflexia (greater than normal reflex activity) in extensors, a spasticity develops. Spasticity is a strong and persisting contraction of either flexors or extensors but not both and should not be confused with rigidity, which is a resistance to movement due to hyperactivity of both flexors and extensors at the same joint. With the spasticity comes the clasp-knife reflex, clonus (rhythmic, alternating contraction and relaxation of a rapidly and briefly stretched muscle), and brisk tendon jerk reflexes.
During spinal shock, the bladder wall is atonic, with increased sphincter tone, resulting in extensive dilatation of the bladder. During recovery, there is a gradual increase in wall tone, and emptying of the bladder may occur with exaggerated flexion reflexes. Vasomotor control (control of blood vessel diameter) is also lost below the transection during spinal shock, and postural hypotension (reduced blood pressure when standing) becomes a problem for the "spinal" patient. During recovery, the automatic control of vessel diameter returns, and the problem disappears.
When the transection occurs at higher spinal cord levels, the control of body temperature is lost. The more of the spinal cord left connected to the brain by a transection, the more temperature regulation returns after spinal shock, presumably because more of the exit pathways from the spinal cord are available. Endocrine function will be impaired to the extent that it is mediated by the autonomic nervous system or requires sensory information carried by the nervous system for its regulation. The more spinal cord there is connected to the brain stem, the more of the thoracicolumbar and craniosacral efferent fibers are available for use in regulating both temperature and endocrine function.
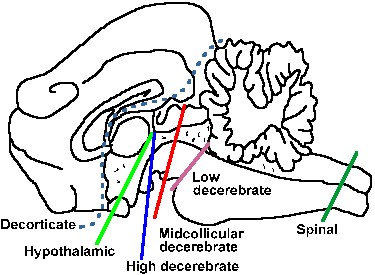 |
Fig. 16-1. The locations of lesions of the neuraxis whose consequences are listed in Table 16-2. Numbers correspond to the numbered lesions in the table. The lesions are drawn on the brain of a cat. |
| Disruptions of the higher neuraxis |
A strikingly different pattern of effects is produced by lesions at higher levels of the central nervous system. Table 16-2 gives a summary of the experimental lesions and their effects. The levels of the lesions are shown in Figure 16-1; the numbers in circles correspond to the numbers in the table. The decerebrate and decorticate conditions are seen in humans. Sectioning the brain stem and certain vascular lesions at the anterior border of the trapezoid body produce what is known as a "low decerebration." The low decerebrate human is usually comatose and shows extensor spasticity as in the later stages of spinal transection, but of more severity. The low decerebrate animal cannot stand by itself despite the heightened extensor tone, partly because it lacks vestibular control to maintain upright posture. It cannot right itself if turned upside down; it cannot walk; and it cannot regulate its temperature or endocrine function.
A slightly higher lesion, at midcollicular levels, produces a "Sherringtonian" decerebration. The lesion in the human is often caused by impact on the vertex as a result of a fall or, commonly, as a result of flying over the handle bars of a motorcycle that has made a sudden stop! Extensor spasticity is extreme in the Sherringtonian patient, causing it to be termed decerebrate rigidity, but in truth the condition is a spasticity. Extensor muscles are in extreme contraction, giving off large amounts of heat, driving the body temperature up, and throwing the person into a posture with the head thrown back and back arched (opisthotonos) and the arms, legs, and digits extended. The Sherringtonian decerebrate animal resembles the low decerebrate animal, except for the severity of the spasticity and the presence of certain postural reflexes. Rotation of the head increases extensor contraction on the side toward which it is turned and decreases it on the other side, but the animal still cannot stand or right itself.
Decerebrate rigidity requires intact dorsal roots. Cutting the roots eliminates the spasticity, producing a flaccid paralysis, but this is not a reasonable treatment, because the spasticity either goes away by itself or the patient never regains consciousness; in either case cut roots would be of no advantage. The low decerebrate condition depends upon activity descending mainly through the lateral vestibulospinal tract from Deiters' nucleus, whereas the Sherringtonian condition also depends upon activity in the pontine reticulospinal tract. Presumably, the lesions that produce the spasticity remove ongoing influences on motoneurons that are net inhibitory influence, exerted upon these tracts by structures above them, allowing them to excite the extensor motoneurons continuously. With the return of function of the higher structures in recovery, the vestibulo- and reticulospinal tracts are somehow brought under control again. Elimination of the spasticity by dorsal root section implies that descending influences are exerted upon gamma-motoneurons, not alpha-motoneurons; thus it is called gamma spasticity. Removal of the cerebellum during gamma spasticity increases the spasticity, but the spasticity is no longer abolished by dorsal root transection, implying that, in this case, the effect is due to strong activation of alpha-motoneurons, i.e., that it is an alpha spasticity.
Brain-stem Control of Movement
| Lesion or preparation | Flaccidity | Extensor spasticity | Stands | Rights | Walks | Temperature, water, endocrine control | Sham rage |
| 1. Spinal | Early in spinal shock | Later after recovery | No | Depends on level | No | Depends on level | No |
| 2. Low decerebrate (bulbospinal)* | No | Yes | Not by itself | No | No | No | No |
| 3. Sherringtonian (midcollicular)* | No | Yes, extreme | Not by itself | Reflexes present, but not full behavior | No | No | No |
| 4. High decerebrate (mesencephalic) | No | Yes, but less than above and not during movement | Yes | Yes | Yes | No | Yes, but less than below |
| 5. Hypothalamic | No | Yes, but less | Yes | Yes | Yes | Yes | Yes |
| 6. Decorticate | Only if decortication is limited to area 4 | Yes, but least | Yes | Yes | Yes | Yes | Yes |
| * Usually comatose in humans | |||||||
Transections above mesencephalic and hypothalamic levels rarely occur in humans, but they can be produced in animals. Extensor spasticity is present following lesions at both levels, but it is less severe when more tissue is left attached to the spinal cord. Animals with experimentally produced lesions at either level can stand unaided, right themselves when inverted, and walk. The high decerebrate or mesencephalic spasticity disappears when the animal moves by itself. The hypothalamic animal regulates its temperature and endocrine function to some extent, because important autonomic centers in the hypothalamus are spared. Both mesencephalic and hypothalamic lesions lead to a phenomenon known as sham rage, which is characterized by biting and hissing when the tail is pinched, but this behavior is not directed toward the offending object (it is thus sham rage).
If the cerebral cortex is destroyed or separated from the rest of the system by a lesion of the internal capsule, a constellation of symptoms similar to that in the animal with a hypothalamic lesion is seen. With large-scale removal of cortex, extensor spasticity develops, but it is less severe than in any of the other conditions. If, on the other hand, the lesion is limited to cortical area 4, the "motor" cortex, flaccidity develops instead of spasticity. Possibly, the reduced severity of the spasticity with lesions at higher levels indicates that the activity of the vestibulospinal and reticulospinal tracts is more inhibited when more tissue is left in connection with them, but there are other tracts that originate at higher levels, e.g., the rubrospinal tract, which could be exerting inhibitory effects directly upon extensor alpha-motoneurons or indirectly upon extensor gamma-motoneurons through excitation of flexor alpha-motoneurons or flexor gamma-motoneurons and reciprocal innervation.
| Supraspinal motor centers |
Many structures above the spinal cord have been referred to as motor structures, and it is to them that we turn our attention. In this section, anatomical and physiological circuits within each structure are discussed, along with current concepts of their role in movement. In addition, clinical manifestations of diseases involving these structures are presented. A later section of this chapter presents a synthesis of the control of movement, involving all of these structures.
The brain stem
Brain-stem pathways usually recognized as motor include those arising from the vestibular nuclei (vestibulospinal), the interstitial nucleus of Cajal (interstitiospinal), the pontine and medullary reticular formation (reticulospinal), and the red nucleus (rubrospinal). In addition to these well-known motor centers, the brain-stem motor systems involve areas of the hypothalamus and the limbic system.
Lesions that selectively interrupt the vestibulo-, interstitio-, and reticulospinal tracts in humans are rare if they ever occur at all, but they can be produced in animals. Such interruptions in monkeys lead to a flexion bias of the trunk and limbs that is manifested in forward slumping in sitting postures, retraction of the shoulders, and slight flexion of the elbow joint. (This picture contrasts sharply with the extensor bias resulting from transection of the neuraxis as discussed previously.) The monkeys stand with a crouched posture, walk with an unsteady gait, and have some impairment of righting ability, at least early after the surgery. They often miss their targets in jumping and show ataxia (a lack of fluidity of movement) involving mainly axial and proximal muscles, i.e., those of the trunk and proximal parts of the extremities. On the other hand, these animals show no impairment of ability to use their hands and fingers.
The effects of rubrospinal tract (and, as we shall see later, also pyramidal tract) interruption are quite different. Following rubrospinal lesions there is no unsteadiness of gait and no proximal ataxia or flexion bias. Rather, the impairments are found in independent finger and hand movements and ability to flex the extended limb. By contrast, animals with rubrospinal tract interruption reach for objects using mainly the proximal arm and trunk muscles, whereas those with vestibulo-, interstitio-, and reticulospinal interruption reach using the distal muscles of the limb more than the proximal muscles.
These observations have led to the suggestion that the ventromedial brain-stem pathways-vestibulo-, interstitio-, and reticulospinal-are especially concerned with maintenance of erect posture and integrated movements of body and limbs, and with directing the course of movement. The lateral brain-stem pathway-rubrospinal-is suggested to be responsible for the capacity for independent use of the extremities, particularly the hand. The corticospinal pathway would then provide a capacity similar to that provided by the rubrospinal tract, but, in addition, would provide for a further independence of movement, especially by individual fingers. As we shall see when discussing the pyramidal tract in detail, the picture of movement control is not quite this simple. The ventromedial brain-stem pathways also exert some influence on distal musculature, and the lateral brain-stem pathway and corticospinal tract also influence proximal musculature. In addition, the foregoing suggestion does not easily explain the small size of the red nucleus and rubrospinal tract in man or the well-developed pyramidal tracts in some animals, notably seals, that do not have independent finger movements, but it serves at least to summarize the main deficiencies that occur when these pathways are interrupted.
Movements can be evoked by electrical stimulation of many sites within the central nervous system. Electrical stimulation of the cerebral neocortex leads to movements of parts of the body or, if suitably small stimuli are used, contractions of individual muscles, but complex behaviors (patterns of movements) are usually not evoked by neocortical stimulation. In contrast, stimulation of certain areas of the brain stem leads to complex motor patterns, involving sequential performance of a variety of movements. This suggests that the brain stem contains "hard-wired circuits," specific, permanent neural connections, for such complex motor patterns. Some examples may help to illustrate the types of motor patterns evoked by brain-stem stimulation. Stimulation within the lateral hypothalamus leads to eating, possibly because it induces sensations of hunger. Electrical stimulation of other points within the lateral hypothalamus leads to nest building, paper gathering, and gnawing (not eating, just chewing, for example on wooden blocks) behavior in rats. Sexual activity in rats can be induced by stimulation of certain sites in the posterior and anterior dorsolateral hypothalamus. Attack and escape behaviors can similarly be elicited by stimulation of regions of the amygdala (the amygdala is cortical tissue, but not neocortical), hypothalamus, and mesencephalon.
It seems likely that these sorts of stereotyped behaviors are permanently "wired" into the nervous system and simply triggered or both triggered and modulated by activity from the peripheral receptors and from the cerebral cortex or elsewhere within the central nervous system. Brief reflection will show that many of these behaviors will fail if not performed properly. It is therefore quite reasonable that they should be governed by relatively immutable neural programs. Exactly where the neural circuits for these behaviors may be localized, if in fact they are localized, is not known.
The cerebellum
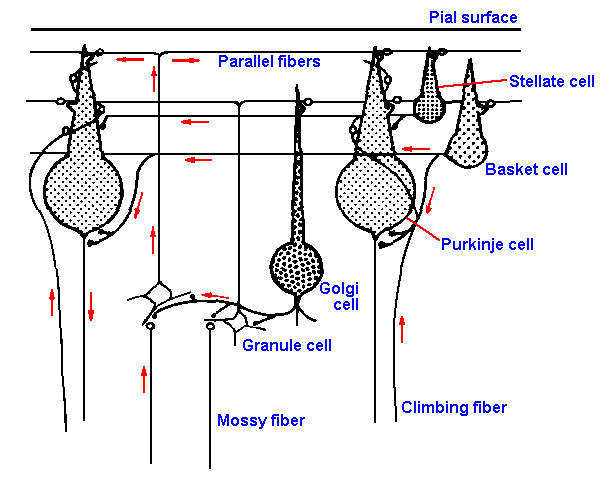 |
Fig. 16-2. Circuit diagram of the cerebellar cortex. Two sources of afferent impulses, mossy and climbing fibers; one efferent cell, Purkinje cell (two are shown); one excitatory interneuron, granule cell; and three inhibitory interneurons, Golgi, basket and stellate cells, are shown. (Allen GI, Tsukahara N: Physiol Rev 54:957-1006, 1974) |
The cerebellum lies over the brain stem and is connected to the medulla by three thick connectives, the inferior, middle, and superior cerebellar peduncles. The outer surface, called the cortex, is highly convoluted and covers a region of white matter, containing inputs and outputs from the cortex, and a region of nuclei: the fastigial, dentate, globose, and emboliform nuclei. (The last two are indistinct in animals other than apes and man and together are termed the nucleus interpositus.) The cortex is divided into three zones: the vermis, the hemisphere, and the flocculonodular lobe. The flocculonodular lobe is completely buried in man; it is phylogenetically the oldest part of the cerebellum, and comparative anatomists say it has changed little throughout its evolution. The vermis constitutes the majority of the cerebellum in most animals, but in apes and man it is quite overshadowed by the hemispheres. Anatomists divide the vermis into many parts; for our purposes it suffices to treat it as a single unit lying along the midline of the cerebellum. The hemispheres, on the other hand, can be usefully divided into an intermediate zone (also called pars intermedia or paravermis), flanking the vermis, and a lateral zone (or pars lateralis).
The cellular structure of the cerebellar cortex is practically identical throughout, and therefore it is possible to draw a circuit diagram for the cerebellum as shown in Figure 16-2. The cortex has two kinds of afferent fibers: the climbing fibers, so named because they ascend into the cortex and climb along the Purkinje cells' dendrites, and the mossy fibers, named for their microscopic appearance. Most (some say all) of the climbing fibers come from the inferior olive, whereas the mossy fibers come from the pontine nuclei, the lateral reticular nucleus, the spinocerebellar tracts, and perhaps elsewhere. There are two "straight-through" pathways in the cerebellar cortex: one involving direct excitation of the output neurons, the Purkinje cells, by the incoming climbing fiber activity, the other involving indirect excitation of Purkinje cells by incoming mossy fiber activity by way of the granule cells and their axons, the parallel fibers. The interneurons of the cerebellar cortex, other than granule cells, are all thought to be inhibitory to Purkinje cells or to the mossy fiber-granule cell relay. These interneurons, called basket cells, Golgi cells, and stellate cells, are excited by mossy fiber activity through the granule cells and perhaps also by climbing fiber activity. As a result of the circuit shown in Figure 16-2, the mossy fiber input excites the Purkinje cells and then inhibits them by a feedforward inhibition (inhibition at a subsequent site in the pathway) mediated by stellate and basket cells and inhibits their subsequent inputs through mossy fibers by a feedback inhibition (inhibition at a previous site in the pathway) mediated by Golgi cells. The only output of the cerebellar cortex is by way of Purkinje cell axons that inhibit their target neurons in the cerebellar nuclei and elsewhere.
 |
 |
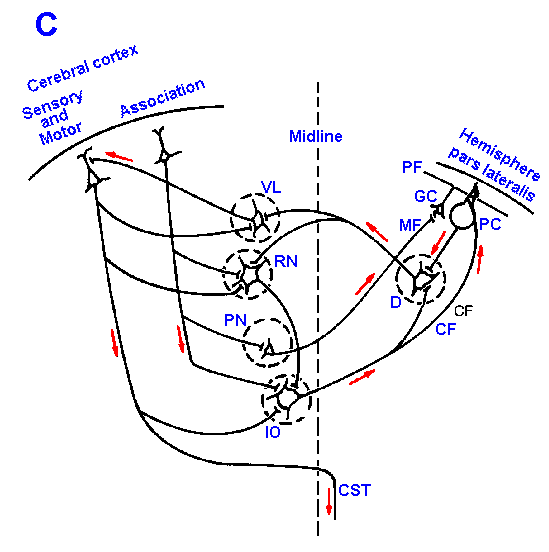 |
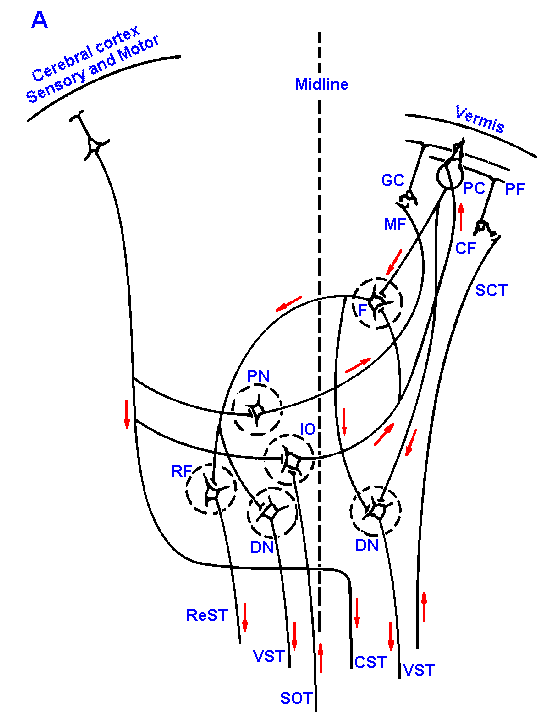
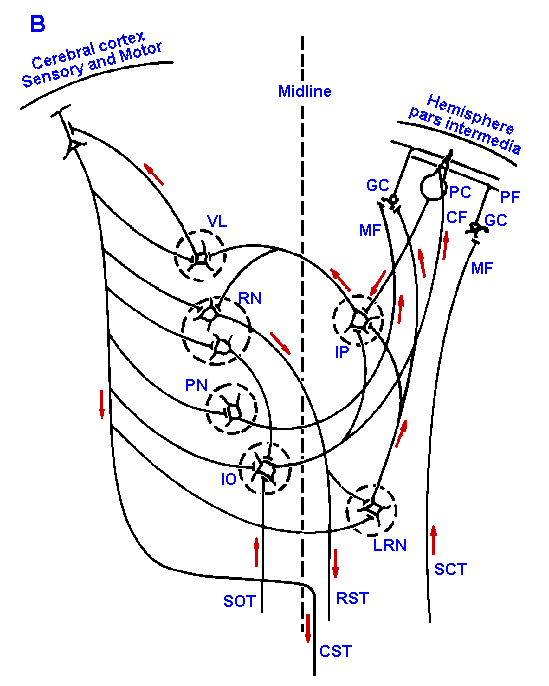
Fig. 16-3. Cellular connections of the cerebellar cortex. Synaptic connections of cerebellar vermis (A), hemisphere, pars intermedia (B), and hemisphere, pars lateralis (C) are shown with the cerebral cortex and descending tracts.Abbreviations used in Fig. 16-3: CF=climbing fiber; CST=corticospinal tract; D=dentate nucleus; DN=Deiters' nucleus; F=fastigial nucleus; GC=granule cell; IO=inferior olive; IP=interpositus nucleus; LRN=lateral reticular nucleus; MF=mossy fiber; PC=Purkinje cell; PF=parallel fiber; PN=pontine nucleus; ReST=reticulospinal tract; RF=reticular formation; RN=red nucleus; RST=rubrospinal tract; SCT=spinocerebellar tracts; SOT=spino-olivary tracts; VL=ventrolateral nucleus of thalamus; VST=vestibulospinal tract |
The major differences between the various portions of the cerebellar cortex are the origin of the mossy fibers that enter them and the destinations of the Purkinje cells leaving them. This is shown schematically in Figure 16-3 for the vermis in A and the hemisphere, pars intermedia in B and pars lateralis in C. Mossy fibers to the vermis originate in the spinocerebellar tracts and the lateral reticular nucleus. The vermal Purkinje cells send their axons to the fastigial nucleus and to Deiter's vestibular nucleus. For comparison, the mossy fibers to the pars intermedia of the hemisphere come from the lateral reticular nucleus, the pontine nuclei, and the spinocerebellar tracts; the pars lateralis receives mossy fibers only from the pontine nuclei. The Purkinje axons of the intermediate lobe project to the globose and emboliform (interpositus) nuclei, whereas those from the lateral lobe project to the dentate nucleus.
The pontine nuclei receive inputs from the cerebral cortex both through collaterals of pyramidal tract axons and through corticopontine fibers. The lateral reticular nucleus receives its inputs from the cerebral cortex, from spinoreticular pathways, from the red nucleus, and the fastigial nucleus. The inferior olive, the source of most climbing fibers, receives its input from the cerebral cortex, the spino-olivary tract, the red nucleus, and perhaps the caudate nucleus.
Because the neuronal circuit is the same throughout the cerebellar cortex, its mode of operation can be indicated using the lateral hemisphere as an example. The same sort of analysis will presumably apply to any of the other components. The pontine fibers, on their way to the lateral cortex, give off a collateral to the dentate nucleus. This is an excitatory connection. In the cortex, the pontine mossy fibers excite granule cells that, in turn, excite (through their axons, the parallel fibers) Purkinje cells and inhibitory interneurons. Thus, the Purkinje cells are first excited and then inhibited. The Purkinje cell output then inhibits the dentate nucleus for a short time. The result, for the cerebellar nuclei, is first excitation (through direct mossy fiber inputs) and then inhibition (through the cortical circuits). The same pattern also holds for climbing fibers: the climbing fiber first excites the nuclear cells, and then they are inhibited by climbing fiber activation of the inhibitory Purkinje cells.
This detailed discussion of cerebellar anatomy and physiology was presented to illustrate the detail in which anatomical connections of parts of the CNS are known and to illustrate how knowledge of the anatomical and physiological relationships can be used to suggest the role of the cerebellum in movement. Table 16-3 presents a summary of the inputs to and outputs from the various parts of the cerebellum. The vermis and the associated fastigial nucleus get their major inputs from the peripheral sensory receptors and send their outputs back to the spinal cord by way of the reticulospinal and vestibulospinal tracts. We have already seen how powerful these outputs can be in decerebrate rigidity. It has been argued that this close connection with the spinal cord motor nuclei make the vermis well suited for making follow-up corrections, corrections necessary to bring the actual movement into line with the intended movement. The hemisphere pars intermedia, on the other hand, gets both cerebral cortical and peripheral sensory inputs and sends outputs back to the cerebral cortex and to the peripheral motor apparatus by way of the rubrospinal and pyramidal tracts. The suggestion has been made that the intermediate lobe of the cerebellum participates in movement correction or modification before the movement is completed. The output through rapidly conducting rubrospinal and corticospinal fibers is consistent with this idea, but fibers in the reticulospinal and vestibulospinal tracts also conduct rapidly.
The pars lateralis of the hemisphere apparently does not receive a significant input from peripheral sensory receptors, instead receiving its inputs from the sensory, motor, and association cerebral cortices. It sends its output, through the dentate nucleus, back to the sensory and motor cortices. The suggestion has been made that the lateral hemispheres work with the association cortex in planning movements rather than in executing and correcting them. There is, however, little or no compelling evidence that either the association cortex or the cerebellar hemispheres actually participate in movement planning. Cooling the dentate nucleus makes monkeys move more slowly with longer delays, require more sensory cues to guide performance, and make more errors. It is not clear to this writer that these changes are necessarily the result of poor planning. They could just represent another example of improper timing. Similar deficits are seen in some patients with parkinsonism, a disease of the basal ganglia.
Clinical manifestations of cerebellar disease are consistent with a role in coordination of muscle contraction and in prediction and correction of motor events in order to achieve proper force, direction, and rate of movement. Decerebellation is apparently without effect on sensation, but the resultin-motor deficits are profound. Patients with lesions in the cerebellar peduncles or with cerebellar degeneration, the most common cause of which is excessive chronic alcohol intake (alcoholic cerebellar degeneration), may show any or all of these symptoms: (1) cerebellar ataxia, (2) hypotonia, (3) asthenia, (4) decomposition of movement, (5) dyssynergia, (6) dysmetria, (7) dysdiadochokinesia, (8) scanning speech, and (9) intention tremor. The patient with cerebellar disease may show ataxia, a lack of fluidity in movement that is manifested in walking as a wide-based gait with staggering and reeling, a characteristic seen also in the acute drunken state. The patient with cerebellar disease has hypotonia, a decrease in muscle tone that shows up as excessive pendular, back-and-forth oscillations of the lower limb when the patellar tendon is tapped, and asthenia, a muscle weakness that is usually moderate. He has impaired cooperation of all muscle groups so that movements normally made up of many parts in a fluid, continuous whole, are broken up into a series of unitary movements. For example, in stepping over an object, the patient may first raise his leg to the correct height, then move it forward past the object, and only then start to put it down. This is decomposition of movement.
A similar abnormality is dyssynergia, a term that refers to lack of cooperation between closely related muscle groups. The usual test for dyssynergia is to have the supine patient touch one knee with the opposite heel and then slide the heel down the leg to the ankle, a movement requiring cooperation of a number of muscles. The cerebellar patient shows a great deal of difficulty doing this. Dysmetria is an abnormality in the range of movement. The patient is unable to start and stop the movement at appropriate times so that he undershoots or overshoots intended targets. The common clinical task for the patient is to touch alternately the examiner's finger and his own nose. It is an easy task for a normal person. Rapid, alternating movements are also impaired with cerebellar disease. Alternate pronation and supination of the hand, for example, done slowly and with difficulty is a common sign, termed dysdiadochokinesia.
Summary of Cerebellar Connections
| Vermis | Hemisphere | Flocculonodular Lobe | ||
| Pars Intermedia | Pars Lateralis | |||
| Source of afferent fibers | Peripheral receptors: via spinocerebellar tracts; via lateral reticular nucleus; via inferior
olive
Sensory and motor cortex: via pontine nuclei |
Peripheral receptors: via spinocerebellar tracts
Sensory and motor cortex: via pontine nuclei, via lateral reticular nucleus; via red nucleus and inferior olive |
Sensory and motor cortex: via pontine nuclei; via inferior olive | Same as vermis |
| Destination of nuclear fibers | Fastigial to reticular nucleus and reticulospinal tract
Fastigial to Deiters' nucleus and vestibulospinal tract Direct Purkinje cell influence on Deiters' nucleus |
Interpositus to thalamus, nucleus VL, sensory and motor cortex and pyramidal tract
Interpositus to red nucleus and rubrospinal tract* |
Dentate to thalamus, nucleus VL, sensory and motor cortex and pyramidal tract
Dentate to red nucleus and rubropsinal tract* |
Direct Purkinje cell influence on all vestibular nuclei |
| * This pathway may not be of great significance because of the reduction in size of the red nucleus in man. | ||||
Patients with cerebellar disease often have difficulties speaking, using irregular volume or speech rhythms, due to inability to control accurately the rate of flow of air past the vocal cords. This is termed scanning speech. In addition, the patient may demonstrate a tremor, but it is subdued, perhaps even unnoticeable, except when the person is actively engaged in some movement. This is intention tremor, which should be distinguished from the tremor at rest of the patient with basal ganglion disease.
Damage to the cerebellum caused by wounds instead of disease, presents a slightly different picture. Surgeons have occasion to remove a part of the lateral aspect of the cerebellum in surgery for tumors of the VIIIth cranial nerve, and they normally find only minor and transient deficiencies. On the other hand, even minimal damage to the dentate nucleus can produce the symptoms described above. Cerebellar symptoms can also be produced by a medulloblastoma, a tumor that arises from the fourth ventricle in children and involves mainly flocculonodular tissue producing disequilibrium, nystagmus (rapid lateral eye movements), and obstruction of cerebrospinal fluid flow. It is difficult, however, in this case to rule out involvement of the deep cerebellar nuclei, which appears to be more detrimental than involvement of the cerebellar cortex alone. It should also be stressed that removal of a section of tissue is quite different from having the tissue present but functioning abnormally. Malfunctioning tissue can be expected to have more severe consequences than the absence of tissue. Perhaps a good analogy is an electronic computer. If you go around behind it and remove a small part, it will probably have a smaller, more predictable, and more easily compensated effect on the functioning of the computer than if you were to stand there and intermittently short-out a part of the circuit.
Apparently, other parts of the nervous system can "fill in" for the cerebellum, because an occasional child is born without one (at least, without the vermis) and without cerebellar symptoms. Whatever the function of the cerebellum is, that function can apparently be assumed by other parts of the nervous system if the cerebellum fails to develop or is damaged in a young child.
Cognitive role of cerebellum
For many years, it was held that the cerebellum had nothing to do with intelligence or learning. Now, it appears that the cerebellum does have something to do with these abilities. When PET scans are used to measure brain activity, both the left frontal cortex (Broca'a area) and the right lateral cerebellum "light up" when subjects are required to identify actions that are associated with certain nouns (e.g., associate bark with dog, fly with kite). A patient with impaired posterior inferior cerebellar artery circulation leading to right cerebellum damage was unable to learn word associations. Solving a peg board puzzle produces greater activation of the dentate nucleus and lateral cerebellum than does simply moving the pegs on the board. The cerebellum, then, appears to be involved in motor learning as well as a least some kinds of verbal learning. What might be its role in the latter is uncertainThe term, basal ganglia, has been applied to a number of structures but is now reserved for six specific structures: the caudate nucleus, putamen, globus pallidus, claustrum, subthalamic nucleus, and substantia nigra. The basal ganglia appear to be part of a neural loop involving cerebral cortex, basal ganglia, thalamus, and cerebral cortex again. The major anatomical connections through the basal ganglia appear to be from the cerebral cortex to the caudate nucleus and putamen, then to the globus pallidus, to the thalamus, and back to the motor and premotor cortex. It is particularly the motor cortex that gets the output from the basal ganglia, but a number of cortical areas provide inputs to the basal ganglia. The globus pallidus is interconnected with both the subthalamic nucleus and the substantia nigra. A dopamine-containing pathway from the substantia nigra to the caudate nucleus and putamen has received attention for its role in parkinsonism. The basal ganglia do not influence alpha-motoneurons directly; their influences on movement are exerted only indirectly through the cerebral cortex. Information from the basal ganglia and the cerebellum could possibly be integrated in the thalamic nucleus ventralis lateralis, which relays activity from both sources to the motor cortex. We do not know what the basal ganglia do, but, because of their connections and the nature of the syndromes associated with them, we say they have some motor function.
The choreas (dancelike movements) are a group of basal ganglion disorders characterized by rapid involuntary movements (dyskinesia), largely restricted to muscles of the distal extremity and face, though others may be involved. These are usually accompanied by a decrease in muscle tone. Huntington's chorea is a devastating disease with progressive dyskinesia and dementia (loss of intellectual function). The disease is inherited (autosomal dominant pattern) and of late onset, usually after the childbearing years. Histologically, the brains of patients with Huntington's chorea show profound loss of neurons and gliosis in the basal ganglia and the cerebral cortex, but this is particularly notable in the caudate nucleus. Evidence has been presented that Huntington's disease is accompanied by a decrease in glutamic acid decarboxylase, the enzyme responsible for converting L-glutamic acid to g-aminobutyric acid, an inhibitory transmitter substance. Whether this reflects a specific enzyme defect or just a reduced amount of transmitter substance due to the reduced cellular population is unknown.
Ballisms or hemiballisms are involuntary movement disorders that are normally unilateral and characterized by violent flailing and swinging of the extremities due to activity in proximal muscles. They are frequently of sudden onset, which may suggest a vascular origin, a suggestion supported by pathological findings of small infarctions in or near the contralateral subthalamic nucleus in patients with ballism.
Unlike the choreiform or ballistic movements, those characterizing athetosis are slow and twisting movements of the extremities including the hands and feet. Pathological examination usually reveals atrophy of the putamen, and there is usually a history of perinatal hypoxia. The hypoxia can sometimes be the result of hemolysis due to Rh or other incompatibility between the blood of the mother and the fetus.
The most common and, therefore, most well-known of the basal ganglia disorders is parkinsonism(1). The patient with Parkinson's disease has true rigidity, in contrast to the spasticities of brain-stem disorders. Both flexors and extensors contract together, and resistance to stretch is present throughout the entire range of passive movement and at any speed of movement. When the rigid muscle is extended passively, it lengthens in steps as if it were a stick being dragged over a series of notches in a piece of wood. This is called cogwheel rigidity. Tremor in parkinsonism usually involves the extremities, but may also involve the face. The tremor is a tremor at rest; the amplitude of the tremor is reduced during active movements, but usually does not disappear. In the hand, the tremor takes the form of the extension of the interphalangeal joints and flexion of the metacarpalphalangeal joints so the fingers beat against the thumb about five times per second.
Patients with parkinsonism usually either do not move much (akinesia) or they move only slowly (bradykinesia). However, their startle responses are normally quite rapid. They lack facial expressions, being described as having mask-like faces, but they can generate a happy or sad face if they are requested to do so. They show postural fixations, in which they assume abnormal postures in spite of their ability to assume normal ones. For example, the posture of a parkinsonian patient normally involves flexion of head and neck, in contrast to the straight head and neck of an unaffected person. The gait of these patients is usually a shuffling one with small steps and a wide stance, without swinging of the arms. Because they cannot make rapid postural reflex movements, most of the patients easily lose their balance, so the wide stance and small steps are understandable.
It is rare that a specific pathological condition can be associated with the onset of parkinsonism. Sometimes the disease develops after (up to 10 years after) cases of encephalitis and is part of the syndromes of both manganese and carbon monoxide poisoning. A reversible form of the disease is induced in some patients by the phenothiazine tranquilizers used in treatment of some psychiatric disorders. A consistent finding is a depigmentation of the substantia nigra that is correlated with a reduction in dopamine concentration. However, the dopamine concentration is highest in the caudate nucleus and putamen and shows largest reductions in these nuclei during parkinsonism. Eighty percent of the dopamine in brain is found in the basal ganglia. It has been suggested that dopamine is an inhibitory transmitter substance released by neurons of the substantia nigra onto caudate neurons. The symptoms of the disease, according to this hypothesis, are the result of the unchecked activity of neurons in the caudate nucleus. However, there are some people who are beginning to doubt that dopamine serves only as an inhibitory transmitter substance in the caudate nucleus.
In spite of this doubt, a frequently used therapeutic measure for parkinsonism has been to administer to the patient extraordinary doses (about 4 grams per day) of L-dopa, the immediate precursor of dopamine. Dopamine is one of those substances that will not cross the blood-brain barrier, but L-dopa will and, once across, it is converted to dopamine. The dosage can be reduced slightly if L-dopa is given with decarboxylase inhibitors. Some patients who were previously unable to take care of themselves have improved significantly following L-dopa treatment. Others have not been helped, and still others have suffered side-effects. Observations over a period of 5 to 6 years suggest that L-dopa treatment does not halt the progression of the disease, but merely treats the symptoms. For many patients this is enough. It is noteworthy that injection of catecholamines produces immediate remission of symptoms in most patients, but it is short-lived.
| Cortical control of movement |
Movements can be initiated by stimulation of the surface of the cerebral cortex in awake humans during surgery, for example, for treatment of epilepsy. This fact is of importance because it defines the electrically excitable motor cortex, or more commonly, just motor cortex. When the cortex is exposed through a small opening in the skull, it all looks pretty much the same, so the position of the motor cortex in the precentral gyrus, identifiable with electrical stimulation, serves as an important landmark for the surgeon.
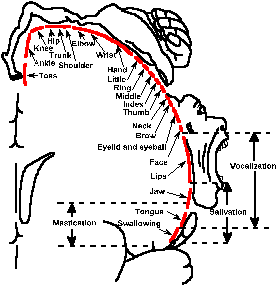 |
Fig. 16-4. The somatotopic representation in the electrically excitable motor cortex. Note the positions and relaive sizes of the representations of different parts of the body on a cross section through the brain at the precentral gyrus. (Penfield W, Rasmussen T: The Cerebral Cortex of Man. New York, Macmillan, 1950) |
When the surface of the motor
cortex is stimulated electrically,
different parts of the body move
depending upon the site of stimulation.
There is a somatotopic motor
representation that resembles the
sensory representation on the other
side of the central sulcus in
somatosensory cortex. Figure 16-4
shows the representation in a cross
section through the brain at the
precentral gyrus. Movements of the
face are elicited by stimulation near the
Sylvian fissure. Slightly more medially
(toward the midline), stimuli evoke
movements of upper extremities, then
the trunk, and finally, on the medial
surface of the hemisphere, the leg and
foot. Notice that the motor
representation of the hand and fingers
is much larger than for the trunk, much as in the sensory representation in the postcentral sensory
representation. This perhaps reflects a greater use and dexterity of the hand.
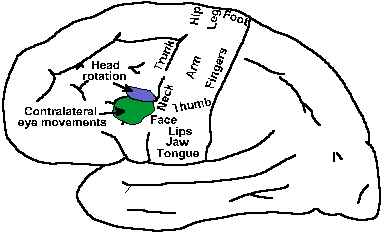 |
Fig. 16-5. A lateral view of the cerebral hemisphere showing the positions of the primary motor area, the frontal eye fields, and the head movement area. (Guyton A: Textbook of Medical Physiology. Philadelphia, WB Saunders, 1975) |
In addition to the precentral or primary motor area, there are other cortical locations from which movements can be evoked by electrical stimulation. Figure 16-5 shows the approximate location of some of these in a lateral view of the cerebral hemisphere. Just anterior to the precentral face representation, on the posterior aspect of the middle frontal gyrus (area 8), lies a part of the cortex from which eye movements can be evoked, the frontal eye fields. The frontal eye fields in humans may extend back at least as far as the lip of the central sulcus. Stimulation in the frontal eye fields typically causes conjugate movements of the eyes toward the opposite side, and ablation produces a fixation of gaze on an object such that a person reports he cannot move his eyes toward a different object without first closing them. Why this strange effect occurs is not known, but apparently this area has something to do with "voluntary" eye movements, in contrast to the more automatic visual tracking. Slightly more medially on the cortex is an area, stimulation of which evokes rotation of the head, again toward the contralateral side. The proximity of this area to the frontal eye fields suggests that they may work together.
Medial to the head rotation area is a motor field called the premotor cortex. This area is sometime divided into 2 subfields, but for our purposes the will be parceled together. Somatotopic maps of the face and extremities also exist in premotor cortex; stimulation here leads to movements involving more than one joint and described as being "more natural." This area and the remaining cortical motor area project to the primary motor cortex and to the spinal cord. In the spinal cord, they project to the same places as pyramidal cells in primary motor cortex.
Not shown in the figure is an area on the medial aspect of the hemisphere just anterior to the lower leg and foot representation of the primary motor cortex. This area of the superior frontal gyrus (together with premotor cortex, comprising area 6) is called the supplementary motor area(1). Stimulation here produces bilateral movements that are one of three types: (1) postural movements, (2) complex patterned movements, and (3) rapid incoordinate movements. Unilateral ablation of this area produces no permanent deficits in either posture or movement; bilateral ablations in animals produce flexor hypertonia, myotatic hyperreflexia, and clonus with some postural deficits.
The function of these areas is thought to be in planning motor activities. The premotor cortex appears to be involved primarily in movements triggered by external stimuli, whereas the supplementary motor cortex is involved primarily in movements initiated by the animal (including humans). Furthermore, the activity of both of these areas is similar in pattern whether the subject is actually performing or simply mentally rehearsing the movement. Finally, the precise premotor area involved in a movement change as the subject better learns the movement, i.e., it changes with practice.
Pyramidal versus extrapyramidal control
Cortical motor systems have traditionally been divided into pyramidal and extrapyramidal systems, but this distinction is overemphasized. To the pyramidal system is normally assigned the function of initiation and control of "voluntary" movements, whereas the extrapyramidal system is said to control the "involuntary" movements, a distinction that is gradually disappearing from major textbooks. First, let us examine the concepts of "voluntary" and "involuntary" movements. Try to think of an involuntary movement! Try a hard one: contraction of the diaphragm, producing inspiration. It goes on night and day without any participation of volition, but it is possible to control the diaphragm and change the breathing rhythm whenever you want to do so. Thus, what was an "involuntary" movement becomes a "voluntary" movement. There is no reason to believe that the neural pathway for ongoing control is different from that in this special circumstance, and certainly the movement itself is no different. From this point of view, it makes no sense to distinguish voluntary and involuntary movements. If it makes no sense to distinguish them, it makes no sense to assign different structures to control them. This is an important idea for our understanding of the organization of the nervous system.There is a distinction that does make some sense in this context. All movements involving the limbs are complex movements. Adduction of the arm not only involves contraction of the arm and shoulder muscles, but also subtle reactions in the axial muscles to compensate for the changes in weight distribution that result from the movement. These postural changes are important components of every movement that are usually overlooked or disregarded. There is considerable evidence that the extrapyramidal tracts (with the exception of the rubrospinal tract) exert their influences most strongly on the proximal or axial musculature, whereas the pyramidal tract exerts its strongest influence on distal musculature. This is perhaps the distinction that should be emphasized.
The pyramidal system
The pyramidal system receives its name from its only constituent, the pyramidal tract, a bundle of fibers on the ventral surface of the medulla, whose cell bodies are located in the pre- and postcentral gyri in areas 1,2,3,4, and also 6. The cells of the pyramidal tract, defined by having an axon in the medullary pyramids, send their axons through the internal capsule and the basis pedunculi on their way to the medullary level. The tract is composed mostly of small fibers, less than one micrometer in diameter, about 1 to 1.5 million in number in man. It has been said that man is what he is because of his pyramidal tract. If this is so, then the human is distinguished by his small pyramids; the human has fewer pyramidal tract fibers per unit of brain than one would expect for a mammal of his or her body size (Towe AL: Brain Behav Evol 7:1-17, 1973).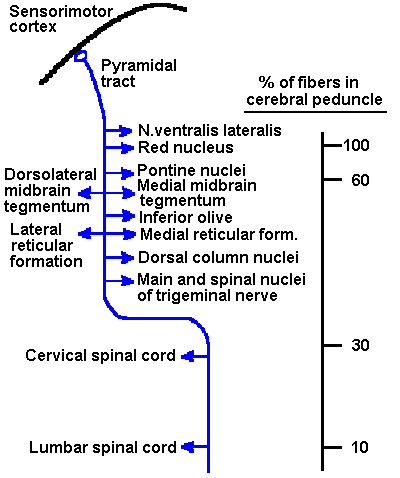 |
Fig. 16-6. The known termination sites of pyramidal tract axons. It is not meant to be implied that a single fiber ends in all of these nuclei; quite the contrary is true. Also indicated is the approximate percentage of fibers in the basis pedunculi that is still present in the tract at various levels. Notice the rapid decline at the pons and also in the brain stem. Not shown are possible terminations in the cranial nerve nuclei in man that do not occur in other animals. |
Some people equate the pyramidal tract with the corticospinal tract, an entirely inappropriate custom because only a fraction of pyramidal tract fibers ever reach the spinal cord. The nature of the terminations of the pyramidal tract is indicated in Figure 16-6(1). Some of the same cells that project into the medullary pyramids also send collaterals into the nucleus ventralis lateralis of the thalamus and the red nucleus before even reaching the pons. Other pyramidal tract fibers terminate in the pontine nuclei, the midbrain tegmentum, inferior olive, lateral and medial reticular formations, dorsal column nuclei, and the main and spinal nucleus of the fifth nerve. In man, though not in the cat, some fibers even end in the motor nuclei of the cranial nerves. If the number of fibers entering the pons is taken as 100%, then only 30% make it as far as the cervical spinal cord and only 10% make it to the lumbar spinal cord. Most of the pyramidal tract fibers end, in fact, in the brain stem, a strange behavior for a tract that is supposed to be the tract mediating "voluntary" movements. (See Towe AL: Motor cortex and the pyramidal system. In Maser JD [ed]: Efferent Organization and Integration of Behavior. New York, Academic Press, 1973 for a thorough discussion of pyramidal tract terminations.)
Look carefully at the distribution of pyramidal tract terminals in Table 16-4. Termination sites are found in as many "sensory" as "motor" nuclei. It is not known why the pyramidal tract has these terminations in sensory nuclei. At the spinal cord level, some pyramidal tract axons end directly on alpha-motoneurons and, thus, exert a direct effect on motor output, but the majority end on interneurons in the dorsal horn. The tract could still play a major role in initiating and controlling movement if it had a powerful excitatory effect on alpha-motoneuron circuits.
Terminations of Pyramidal Tract Fibers
|
|
|
CEREBELLUM
| |
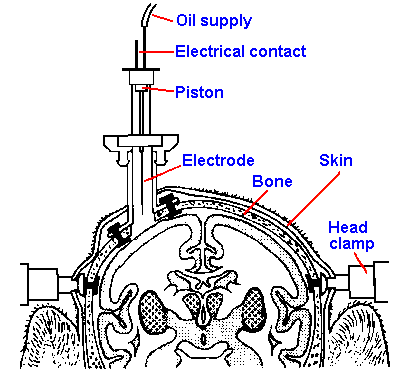 |
Fig. 16-7. The physical setup for recording from awake, moving monkeys. A chamber is attached to the skull in which a small hole has been made. The electrode and its drive mechanism are attached to the chamber, and the electrode, usually a sharpened tungsten or platinum-alloy wire, is driven through the meninges to the vicinity of a single cell. Note that the head is fixed during recording. (Evarts EV: Sci Amer 229:96-103, 1973) |
It is true that stimulation of the precentral cortex elicits movements (by definition of electrically excitable motor cortex), but it requires several (4-5) stimuli, applied at high frequency (500/sec), to obtain the movement through the pyramidal tract. The question is, Do pyramidal tract fibers fire at these high frequencies during movements? They should if they initiate movements by themselves. It is possible to record from single pyramidal tract neurons in an awake monkey, who is free to move, at least in a limited way. Figure 16-7 shows how a chamber is fixed over the recording site and how the electrode is driven into the cortex. A small hole is made in the skull within the chamber, and a tungsten or platinum-alloy wire recording electrode is driven through it. The monkey is seated in a chair and able to move a lever in a certain way in order to get a fruit juice reward, but he is unable to move his head (Fig. 16-8). The neuron's activity is recorded at the same time as the monkey is moving the lever. Figure 16-9 shows the records obtained in this experiment. The upper trace is a recording of the EMG from a muscle involved in the movement, the middle trace is the cell's response, and the lower trace shows the time of movement of the lever, which occurs 3 times in this record. Notice that the muscle begins to contract before the lever moves and the neuron begins to discharge before the movement occurs and even before the muscle begins to contract, but does not generate a train of spikes at 500/sec or more. In fact, if you generate a train of stimuli that matches the timing of the discharges in the figure and apply it to the cortex, no movement will be evoked.
The pyramidal tract does have special properties. Figure 16-10B shows a recording, from inside an alpha-motoneuron, of the EPSP evoked by a single pyramidal tract stimulus. If two such stimuli, S1 and S2, are applied at an interval of 10 msec or less (in the figure, 6 msec), the resulting EPSP is larger than would be predicted from simple temporal summation (Fig. 16-10A). This phenomenon is called facilitation and appears to be a special property of pyramidal tract synapses on alpha-motoneurons. For simple temporal summation, R2 would be less than or equal to R1; for facilitation, R2 is greater than R1. By contrast, the EPSPs evoked by group Ia afferent fibers from muscle spindles do not show facilitation in alpha-motoneurons. This is shown in Figure 16-11A for stimuli at 5-msec intervals applied to the dorsal root; there is no increment in successive EPSPs recorded from a motoneuron of the median nerve. Compare this pattern with the facilitation of corticomotoneuronal EPSPs shown in Figure 16-11B for the same cell(2). Clearly, each successive EPSP in the series of six is larger. What good is facilitation?
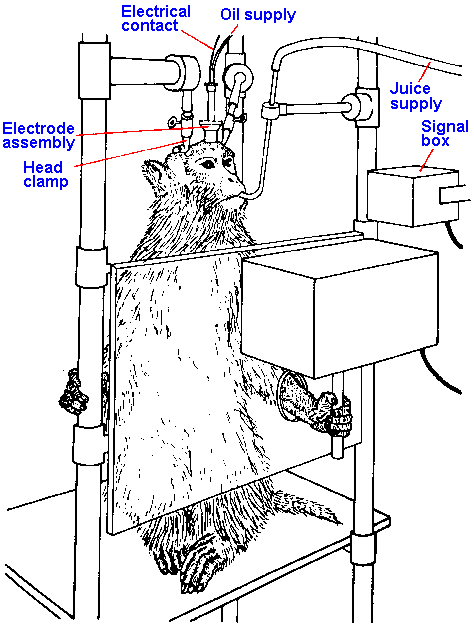 |
Fig. 16-8. A typical primate chair in which the monkey sits unrestrained except at the head. The hand can reach through a hole to grasp a lever. When the monkey moves the lever in a particular way a reward of a small amount of fruit juice is delivered through the tube near its mouth. (Evarts EV: Sci Amer 229:96-103, 1973) |
It appears that no one group of fibers is capable of bringing a motoneuron to the critical firing level by itself in normal functioning of the system. We have just seen that the pyramidal tract cannot do it, and we saw before that in spinal shock even group Ia afferent fibers cannot do it. The critical firing level is achieved by summing all the inputs over different pathways. Figure 16-12 illustrates the positions of the important descending tracts in the spinal cord and the way in which the dendrites of alpha-motoneurons "reach out" toward them. Distal muscles are influenced most strongly by pyramidal and rubrospinal fibers, whereas proximal muscles are influenced most strongly by vestibulo- and reticulospinal fibers, though both groups of muscles are influenced from all four tracts. There is even evidence that different inputs to a single cell may come in on their own dedicated dendrites.
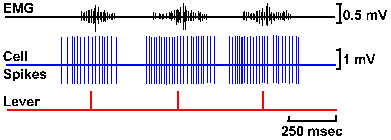 |
Fig. 16-9. Records from a pyramidal tract neuron in an awake monkey. The upper trace shows the EMG recorded from one of the muscles the monkey uses to move the lever. Notice that the amplitude of the EMG record increases just before and while the lever is moved in three separate trials as indicated in the bottom trace. The cell begins discharging (middle trace) long before the EMG record shows any change. Notice that the interval between action potentials within any one of the three trials first decreases to some minimum value and then increases again, but never reaches 2 msec (equivalent to a frequency of 500/sec). (Porter R, Lewis M McD, Horne M: Brain Res 34:99-113, 1971) |
What then is the pyramidal tract doing? Again, no one knows for certain, but consider this hypothesis: The pyramidal tract tells the muscles the precise time when they should begin to contract. Figure 16-13 provides a closer look at the discharge of a pyramidal tract cell during a movement. Notice that there is one interspike interval (the time between two spikes) in the train that is less than 10 msec in duration, the maximum time over which facilitation occurs. In every such train of spikes from a pyramidal tract cell, there is always one interval in this range of durations, so there will always be facilitation at some motoneurons. If all the other inputs to the alpha-motoneuron have brought it near the critical firing level, then the addition of this more powerful (by facilitation) input could drive it over the critical firing level. It turns out that there is a rather high correlation between the time of occurrence of this short interspike interval and the time of onset of the movement. This would seem to support the hypothesis. The time when the pyramidal tract fiber discharges at this short interval may signal the muscle to start the contraction. This is undoubtedly not the only function of the pyramidal tract, but it may be one of them.
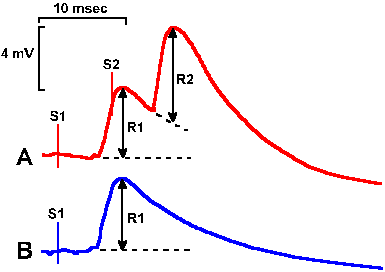 |
Fig. 16-10. Intracellular recordings of facilitation of a corticomotoneuronal EPSP in an alpha-motoneuron. Two identical stimuli, S1 and S2, were delivered to the cortex about 6 msec apart; the resulting EPSP is shown in A. When only S1 was delivered, the EPSP was as shown in B. Notice the facilitation (R2R1), not summation (R2=R1). (Porter R: J Physiol (Lond) 207:733-745, 1970) |
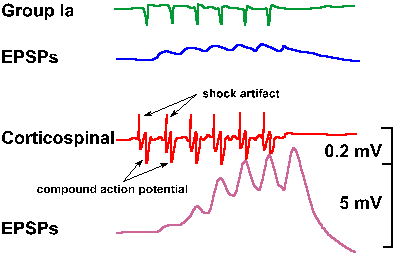 |
Fig. 16-11. Intracellular recordings from a motoneuron of the median nerve. A. Simultaneous records of dorsal root volleys evoked by stimulation of muscle afferent fibers at 200/sec (upper trace) and monosynaptic EPSPs in the motoneuron. B. Simultaneous records of corticospinal volleys at 200/sec (upper trace) and monosynaptic EPSPs on the motoneuron. Cortical stimulus strength was adjusted to give an EPSP the same size as the group I EPSP in A. (Phillips CG: Proc Roy Soc B 173:141-174, 1969) |
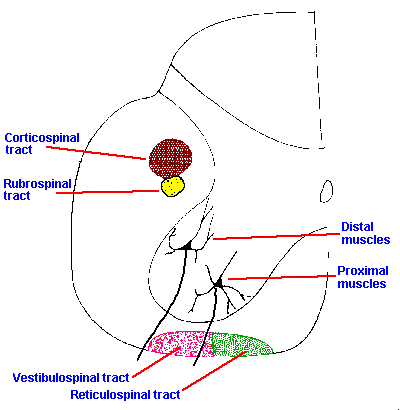 |
Fig. 16-12. A cross section through the lumbar spinal cord indicating the approximate positions of the cortico-, rubro-, vestibulo-, and reticulospinal tracts and the approximate positions of the motor cell columns for proximal and distal muscles. |
With a pyramidal tract lesion or, alternatively, an appropriately placed area 4 cortical lesion, a flaccid paresis of the upper limb results in monkeys. A similar effect also occurs following limited stroke in man. Under these circumstances, the limb usually dangles at the side and is not used for purposive movements; however, if the other, unaffected, limb is immobilized or if a threat can only be warded off with the affected limb, or in certain training situations that the animal has been exposed to before the lesion, then the affected limb can make required responses. The movements are by no means normal-they lack precision, they are clumsy, and they are not guided throughout their entire course. It is interesting that a complete unilateral cervical dorsal rhizotomy has almost the same effect on the use of the ipsilateral upper limb in monkeys. The affected arm is dangled at the side and unused except when forced or in certain training situations and, under those circumstances where it is used, the movements are clumsy, imprecise, and not fully guided. Yet in the former case the loss is usually described as motor, and in the latter case it is usually described as sensory. Perhaps these results best serve to illustrate our lack of adequate understanding of the distinction between sensory and motor activity, or perhaps the nervous system makes no such distinction.
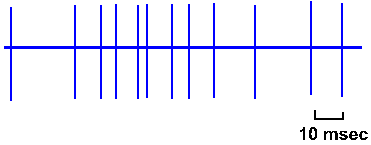 |
Fig. 16-13. An enlarged record of the discharge of a pyramidal tract cell during a movement of the lever by the monkey as shown in Fig. 16-9. (Porter R, Lewis M McD, Horne M: Brain Res 34:99-113, 1971) |
Recovery of movements following pyramidotomy has also varied in different experiments, probably for the same reasons as the variation in initial deficit. The terms, descriptive of the recovery, have ranged from "none" to "almost complete." In fact, in cases of verified, complete pyramidotomy, recovery may be almost complete, with the only lingering signs, a slight paresis and a loss of fluidity and ease of movement, and even the paresis may disappear with time. In general, the permanent losses appear to be in fine-tuning of movements, particularly those involving the distal musculature.
The extrapyramidal system
The pyramidal system is defined in terms of the pyramids, whereas the extrapyramidal system is defined by default in that anything that is not pyramidal is extrapyramidal. Usually, the red nucleus and rubrospinal tract, the pontine and medullary reticular formations and the reticulospinal tracts, the lateral and medial vestibular nuclei and the vestibulospinal tracts, and the tectum and tectospinal tract are included as part of the extrapyramidal system along with fibers originating in the cerebral cortex but not part of the pyramidal tract. Often the basal ganglia are included, but this is not appropriate, because they have no real outlet to the spinal cord except by way of one of these other pathways or the corticospinal tract. Most of the cortical elements that synaptically activate these extrapyramidal centers have no direct connection with alpha-motoneurons.The distinction between pyramidal and extrapyramidal systems is purely artificial. Table 16-4 shows that pyramidal tract fibers have terminations in many extrapyramidal structures, and it is known that they have profound effects there. The pyramidal tract also indirectly influences all of those extrapyramidal structures that it does not influence through direct connections. The systems are clearly inseparable at the level of the cortex, the internal capsule, the basis pedunculi, and certainly at the pons. It is only in the pyramids themselves where a clear separation occurs.
| Upper and lower motoneuron disease |
The only ways to study extrapyramidal effects are to attempt to make lesions in the individual nuclei of the system or to compare the effects of damage to the cerebral cortex with those of pyramidal tract section. The resulting deficits from lesions in some extrapyramidal nuclei were discussed previously.
Clinicians like to refer to upper motoneuron symptoms and lower motoneuron symptoms in motor deficits. The lower motoneurons are, of course, the alpha-motoneurons. The upper motoneurons, in this context, are all the neurons of descending tracts whose activity impinges upon alpha-motoneurons. We may take this as both pyramidal and extrapyramidal influences together. The characteristic symptoms of both upper and lower motoneuron disease are listed in Table 16-5 along with the symptoms of experimental pyramidal tract section. Note that most of the symptoms of upper motoneuron disease are not symptoms seen in controlled pyramidal tract section and are thus assignable to extrapyramidal tracts alone or to some interaction between pyramidal and extrapyramidal tracts. These effects are more impairing and more permanent than those resulting from pyramidal tract section alone.
It is possible to have both upper and lower motoneuron symptoms at the same time. For example, a lesion in the region of the cervical enlargement of the spinal cord that damages the cervical motoneurons and the descending fibers of the vestibulo- and reticulo-spinal tracts in the ventral white matter, will produce lower motoneuron symptoms, including fasciculations and atrophy, in the upper limbs and upper motoneuron symptoms, including Babinski's sign, in the lower limbs. Of course, the cervical motoneurons are also separated from supraspinal influences, but the lower motoneuron symptoms are the only ones seen. Functional disconnection of the muscle from the nervous system is much more devastating for movement than partial denervation of its motoneurons.
Comparison of Upper and Lower Motoneuron and Pyramidal Tract Symptoms
Upper motoneuron disease |
Lower motoneuron disease |
Pyramidal tract section |
| Paralysis or paresis | Paralysis | Paresis |
| Spasticity | Flaccidity | Flaccidity |
| Hyperreflexia | Areflexia for that muscle | Hyporeflexia |
| Babinski sign | None | Babinski sign |
| Clonus | None | None |
| Absence of abdominal reflexes | Depends on motoneurons affected | Absence of abdominal reflexes |
| None | Fasciculations | None |
| None | Atrophy | None |
It is important to note that Table 16-5 lists symptoms of upper motoneuron disease. The results of ablation of the cortex may be quite different. Recall that ablation limited to area 4 produces flaccidity, not spasticity. An even more striking effect occurs in those rare cases in which an entire hemisphere was removed right down to the thalamus. This procedure produces no obvious motor deficits in the clinical sense, but one patient with superior athletic skills preoperatively, did not have such skills postoperatively. This apparent enigma may be resolvable by conjuring up the computer analogy again. The more "abnormal" tissue there is present, the more the abnormality in movement. Complete removal is far less devastating than partial removal. It may be useful in the clinical setting to have committed Table 16-6 to memory. It gives some useful tools for distinguishing between possible levels of lesions involving motor deficits.
Summary of Clinical Signs of Damage at Different Sites Along the Neuraxis
Lesion site |
Reflexes |
Muscle tone |
Spasticity |
Rigidity |
Flaccidity |
Additional clues |
Spinal-shock phase |
Areflexia | Atonic | x | Postural hypotension, atonic bladder | ||
Spinal-recovery phase |
Hyperreflexia | Hypertonic | x | Clonus | ||
Trapezoid body-low decerebrate |
Hyperreflexia | Hypertonic | x | May lack doll's eyes | ||
Midcollicular-Sherringtonian |
Hyperreflexia | Hypertonic | x | May lack doll's eyes, postural reflexes present, comatose | ||
Mesencephalic-high decerebrate |
Hyperreflexia | Hypertonic | x | Conscious, may lack light reflex | ||
Hypothalamic |
Hyperreflexia | Hypertonic | x | Conscious, may lack light reflex | ||
Decorticate |
Hyperreflexia | Hypertonic | x | Clonus, Babinski | ||
Motor cortex-area 4 only |
Hyporeflexia | Hypotonic | x | Babinski | ||
Pyramidal tract |
Hyporeflexia | Hypotonic | x | Babinski | ||
Cerebellar |
Hyporeflexia | Hypotonic | x | Ataxia, intention tremor, dyssynergia | ||
Basal ganglion |
Hyperreflexia | Hypertonic | x | Bradykinesia, tremor at rest (hyperkinesia with ballism) |
| Correlates of movement |
Experiments have begun to show what various parts of the central motor systems are doing when animals are actually moving. A cat can walk almost normally on a flat, horizontal surface after removal of the forebrain and rostral thalamus. As we have already noted, there is no spontaneous walking in a cat when the brain stem is sectioned, but, if the section is made rostral to the superior colliculus and the animal is stimulated in the region of the nucleus cuneaformis, the animal walks, maintaining its equilibrium. The stimulus used is a continuous train of shocks, but the movement is an alternation in the limbs at 1 to 2 Hz. The head and spinal column of the animal can be rigidly fixed because sectioning the brain stem deprives the animal of nociception. Thus, recordings can be made of the activity of any remaining neurons while the animal is walking.
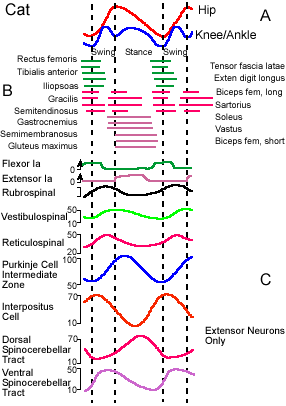 |
Fig. 16-14. The stepping cycle in a mesencephalic cat. A. Movements of the hip, knee, and ankle during the swing and stance phases of stepping. Flexion is indicated by an upward deflection of the curve; extension, by a downward deflection. B. Contractions of various muscles during the steppng cycle. Time of contraction is indicated by the position of the horizontal bar. C. Activity of neurons in various parts of the motor system during stepping cycle. Frequency of discharge is indicated on the ordinates. (Orlovsky GN, Shik ML: Control of locomotion: A neurophysiological analysis of the cat locomotor system. In Porter R [ed]: International Review of Physiology. Vol. 10, Neurophysiology II. Baltimore, University Press, 1976) |
Walking in the mesencephalic cat, a cat with its brain stem sectioned rostral to the superior colliculus, is very close to normal in terms of joint movements and muscle activity, and, like in normal walking, the movement of any limb can be parcelled into a stance phase and a swing phase. The stance phase begins when the foot touches the ground and ends when it is removed from the ground. During the stance phase, the limb exerts a force on the substrate, the horizontal component of which propels the animal forward. The swing phase begins when the animal removes its foot from the substrate and ends when it replaces it. During the interim between these two activities, the limb is lifted and swung forward to be in position to contact the ground for the next stance phase. The sequence is reminiscent of walking in humans as described in Chapter II, except that the knee and ankle of the cat move in phase, the knee and ankle of man do not. As in humans, the movements of the hind limbs of the cat alternate, but because the cat is quadrupedal the forelimbs also participate in locomotion.
Movements of the joints in the mesencephalic cat are shown schematically in Figure 16-14A. In this figure, the joints are flexing when the traces deflect upward, extending when they deflect downward. The upper trace shows movements of the hip joint and the lower trace, movements of the ankle and knee. The latter two joints are plotted together because they move in phase in the cat. The hip joint is extending throughout the stance phase, and the hip and leg are moving backwards relative to the trunk. The knee and ankle are flexing during the first half of the stance phase as they yield to body weight. At the end of the stance phase, all three joints are extending because of active extensor muscle contractions and forward movement of the animal.
The swing phase begins when the limb reaches the most caudal position, with activity in both flexors and two-joint muscles, resulting in flexion of all three joints. Flexion terminates earlier in the knee and ankle, and they begin to extend before the hip. The sequence of muscle activity can be seen in relationship to the stepping cycle in Figure 16-14B. In this figure, the muscle is identified at the left and its action is indicated at the right. The horizontal lines indicate the times when the EMG activity for the muscle increases above its resting level. Many limb extensor muscles are strongly activated before the limb touches the ground. The fact that extensor activity occurs before the footfall implies that it is not a reflex elicited by foot contact. The shortest latency from receptor activation to onset of a reflex change in tension in ankle extensor muscles is about 30 msec. Considerably longer might be required to balance tension among motor units, exceeding the durations of the various phases of the step cycle at least at fast rates of locomotion. Therefore, it is unlikely that reflexes play an important role.
Stepping, in the mesencephalic cat, continues as long as the stimulation continues (a train of pulses is used to drive stepping). Increasing the strength of stimulation results in increased stepping frequency due to increased force developed by the limb (apparently through recruitment of additional motoneurons rather than through increasing motoneuron firing rate), but does not change the phase relationships of the joint movements or the muscle contractions. Changing the frequency of pulses within the train has little effect. Walking or trotting in the cat is characterized by alternating movements in both fore- and hind limbs with each forelimb moving in phase with the contralateral hind limb. As the strength of stimulation increases, the animal changes from a walk to a trot and, with even higher strengths, the animal gallops, with both hind limbs moving in phase and alternating with both forelimbs. This same sequence can be observed in an intact cat, moving spontaneously.
It should be emphasized that this region of the brain stem, the region whose stimulation leads to stepping, is not required for stepping to occur; animals with spinal cord transections are also capable of walking under certain circumstances. This implies that the stepping cycle itself is generated within the spinal cord. The alternation of limb movements does not depend upon crossed reflexes because one limb will continue to step if the contralateral ventral roots are cut, and alternating activity can still be recorded from the ventral roots when ventral roots on both sides are cut distal to the recording site. Dorsal roots are likewise not required for stepping, but after dorsal rhizotomy limb movements are not adapted to the real speed of locomotion (when the animal is on a moving treadmill), and the limbs do not change the force they develop when the load is changed. Thus, although stepping can occur without sensory feedback, the feedback does play a role in normal walking. There are, however, movements in which feedback is not only not required but is not desirable. Ballistic or rapid, pendular movements (such as removing the hand rapidly from a telegraph key) are performed more rapidly without sensory feedback. Sensations from the moving part of the body are greatly reduced just before and during such a movement (Luschei ES: Personal communication). In fact, there would not be time for the information to be used during the movement anyway.
Many of the spinal cord reflexes discussed in Chapter 15 are either modified or eliminated during stepping. For example, Renshaw inhibition, feedback inhibition from collaterals of alpha-motoneurons resulting in decreased frequency of discharge in neighboring alpha-motoneurons, disappears when stepping begins. Likewise, reciprocal inhibition can only be observed at certain appropriate times during the stepping cycle. Thus, stimulation of group Ia fibers of the antagonist muscle will only inhibit the agonist motoneurons when they would normally be silent (their muscle relaxed) during the cycle (in Fig. 16-14B, when there is no horizontal bar).
The behavior of muscle spindle afferent fibers during stepping is also a bit surprising. These muscle receptors are frequently silent as their muscle is being lengthened, but they discharge vigorously as the muscle is actively shortening. This can be seen in the upper two traces of Figure 16-14C(3). The activity of flexor Ia fibers increases at the beginning of the swing phase when most flexors are actively contracting; the extensor Ia discharge increases early in the stance phase when extensor muscles are contracting. This behavior is surprising because we are accustomed to thinking of muscle spindle afferent fibers as length detectors; however, in this case they increase their discharge when the muscle shortens. The observed behavior of the receptors could occur as a result of alpha-gamma coactivation and, in fact, gamma-motoneurons are active at the same time during the stepping cycle as the homonymous alpha-motoneurons. The increased sensitivity of the spindle receptors during muscle contraction allows their activity to influence the homonymous alpha-motoneuron when it is active, whereas the withdrawal of gamma-motoneuron activity during the relaxation phase for the muscle prevents the alpha-motoneuron from being activated by the group Ia afferent input during the wrong phase, i.e., when the muscle is being passively stretched. The Golgi tendon organs are more active during the isometric or lengthening contractions of the stance phase than in the passive stretches of other phases. Therefore, they discharge together with group Ia fibers from extensor muscles.
Similarly, supraspinal descending influences on alpha-motoneurons are variable during the stepping cycle. The reticulospinal tract can be divided into a lateral and a medial division. The effect of the lateral division is excitatory to extensors and inhibitory to flexors, whereas the medial division has mixed effects, most frequently inhibtion of exensors and excitation of flexors. At rest, i.e., when the animal is not walking, the vestibulospinal tract exerts an excitatory action on extensor muscles and an inhibitory action on flexor muscles and the rubrospinal and corticospinal tracts excite flexors. (These are not the only actions of these tracts, but they are statistical statements of the most frequently observed effects.) During the stepping cycle, stimulation of Deiters' nucleus leads to an increase in EMG activity of extensor muscles, but only during the phase of the cycle when they would normally be active, i.e., in the stance phase. Stimulation of the pontine reticulospinal tract leads to increased flexor EMGs and decreased extensor EMGs, whereas stimulation of rubrospinal and corticospinal tracts leads mainly to increased flexor EMGs. These influences are also only exerted during the appropriate time during the stepping cycle.
The neurons in many parts of the central nervous system show rhythmic changes in their discharge frequencies in phase with stepping. Some of these changes are illustrated in Figure 16-14C. Many rubrospinal tract neurons increase their firing rates during the swing phase and decrease their rates during the stance phase. Similar changes are also seen in reticulospinal neurons. Vestibulospinal neurons begin increasing their discharge rates at the beginning of the swing phase, but peak rates occur at the transition from swing to stance and early in the stance phase. These changes in activity of tract neurons are consistent with influences discussed in the last paragraph; the neurons increase their discharges when the target motoneurons are active during the step cycle. It is interesting that this rhythmic variation in discharge rate disappears if the cerebellum is removed, even though stepping continues. Apparently, the modulation in discharge rate reflects rhythmic input from the cerebellum.
In the cerebellar intermediate lobe, Purkinje cells show similar rhythmic changes in discharge rate, and these changes are reflected in discharge of cerebellar nuclear neurons. Both changes are shown in Figure 16-14C. Notice that the changes in the cortex and nuclei are out of phase, as expected from the inhibitory influences of Purkinje cells on nuclear neurons. We have seen that rhythmic changes in descending pathways are due to rhythmic changes in the discharges of cerebellar neurons. The latter changes reflect similar rhythmic changes in spinocerebellar tracts. Such changes are indicated in the lower two traces of Figure 16-14C. The dorsal spinocerebellar tract receives inputs from muscle spindle receptors among other receptors, and rhythmic activity in the tract neurons simply reflects rhythmic changes in receptor activity (compare the dorsal spinocerebellar tract rhythm with that for extensor Ia fibers). The ventral spinocerebellar tract rhythm probably reflects rhythmic inputs from the spinal cord stepping generator mechanism because the rhythms are not disturbed by deafferentation of the limb.
In summary, there appears to be an automatic system for control of locomotion, located within the spinal cord, that can be set into motion by descending impulses and under certain circumstances by impulses from sensory receptors. Presumably, this system is also used in locomotion of the intact animal and perhaps, with appropriate modification for the different gait pattern, in man. This automatic system works by modification of basic reflex mechanisms and alteration of at least some sensory mechanisms. Descending influences in mesen-cephalic animals operate to strengthen contractions of certain muscles in certain phases of stepping, but do not change the basic locomotor pattern. Presumably, in the intact system, descending influences also modify the pattern to compensate for changes in elevation or contour of the substrate and for changes in the goal of the movement, e.g., deviation of the target.
| The initiation of movement |
One can criticize the preceding experiments on grounds that the locomotion was artificially driven by the experimenter, not generated by the animal. It is true that the experiments tell us nothing about how the animal initiates movements or where in the central nervous system they might begin. A very old idea suggests that movements are conceived in the association cortex, which relays the command for the movement to the motor cortex. The motor cortex relays the command to the muscles and at the same time to the cerebellum and basal ganglia. These structures generate corrections or commands for subsequent movements, based in part upon sensory information from receptors, and feed these back to the motor cortex. In addition, sensory information is conducted to sensory cortex and relayed to association cortex for use in generating future movements. A newer idea, discussed previously, suggests that movements are planned or programmed by the association cortex with the cooperation of the lateral cerebellar hemisphere, premotor cortices and the basal ganglia. The plan is then fed to the motor cortex, which issues the command for the movement. The nucleus interpositus updates the movement based upon its continuous monitoring of current limb position and velocity. The interpositus update lags slightly behind the corticospinal command in reaching the motoneurons and modifies the cortically designated motor discharge. As a result of the movement that occurs, sensory signals are generated in receptors and conducted to the cortex, cerebellum, and elsewhere to be used in generating subsequent movements or corrections.
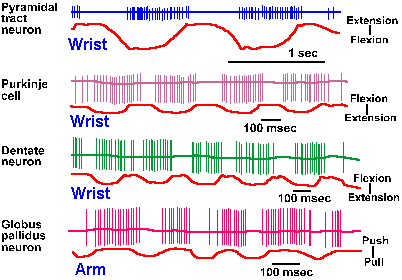 |
Fig. 16-15. Discharges of neurons in motor centers during wrist or arm movements in monkeys. Spikes were recorded in chronic preparations from a pyramidal tract cell, a Purkinge cell, a dentate nucleus cell, and a globus pallidus cell, the first three during wrist flexion and extension and the fourth during push and pull with the arm. The upper trace in each pair is the spike record; the lower trace is a monitor of the movement. (Data from Evarts EV: J Neurophysiol 31:14-27, 1968; Thach W: The behavior of Purkinje and cerebellar nuclear cells during two types of voluntary arm movements in the monkey. In Fields WS, Willis WD [eds]: Cerebellum in Health and Disease. St. Louis, Warren Green, 1970; DeLong MR: Brain Res 40:127-135, 1972) |
Using the chronic recording setup shown in Figures 16-7 and 16-8, recordings have been made in various motor regions in an attempt to find out how the neurons discharge in relation to movements. Unfortunately, studies of the responses of neurons in association cortex have not emphasized the timing of the discharges with respect to the start of the movements. Some neurons in parietal areas 5 and 7 of monkeys do fire before movements of the hand, some up to 400 msec before. Some of these neurons do not appear to respond to sensory stimuli, but discharge at high rates when the animal attempts to reach for an object. Apparently, the same movements performed without a goal (an object) are not accompanied by firing in these neurons. Just what the relationship between these neurons and the movements might be is still to be worked out.
At other places in the central motor system the time relationships of neuron discharges and some movements are better known. Figure 16-15 shows recordings from a pyramidal tract neuron in a monkey's motor cortex, a Purkinje cell in the cerebellar hemisphere, a neuron in the dentate nucleus, and a neuron in the globus pallidus of the basal ganglia. The cell's discharge is shown in the upper trace of each pair and the movement of the handle in the lower trace. The movement in the upper three records was a flexion or extension of the wrist. In the lower trace, the monkey pushed or pulled the lever. Note that each of these neurons fires in relation to a particular phase of a repeated movement of a handle by the monkey. The pyramidal tract neuron begins firing before the animal starts a movement of the handle requiring wrist extension; other pyramidal tract neurons might fire before flexion. Many neurons in the motor cortex, the cerebellar cortex and nuclei, the thalamus, and the red nucleus begin to discharge, increase their discharge rates, or decrease their discharge rates before movements. The discharges in the basal ganglia are usually not related to movement, but a few cells do discharge in relation to movements. The movement-related discharges in motor cortex and the dentate nucleus appear to be related to (1) the pattern of muscular activity required to hold the limb in a certain position, (2) the angle of a particular joint, or (3) the direction of the intended next movement. Neurons in the nucleus interpositus of the cerebellum appear to discharge in relationship only to the pattern of muscular activity [number (1) above].
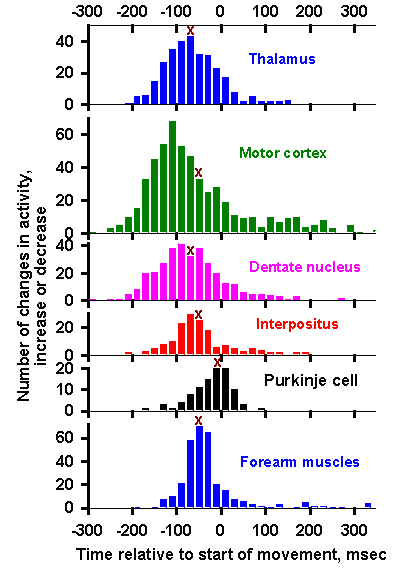 |
Fig. 16-16. Discharges of neurons in motor centers near the time of a movement. The time of the first change (either increase or decrease) in the discharge frequency of the neurons is plotted relative to the start of a movement. On the ordinate is the number of times a neuron was observed to change its discharge at each time before (time in negative numbers) and after (time in positive numbers) the movement. Movement began at time zero. The arrow above each histogram shows the median time of the first change in discharge for neurons in that center. The lower record shows the same kind of plot indicating when forearm muscles began to discharge. (Data from Evarts EV: J Neurophysiol 31:14-27, 1968; Thach W: The behavior of Purkinje and cerebellar nuclear cells during two types of voluntary arm movements in the monkey. In Fields WS, Willis WD [eds]: Cerebellum in Health and Disease. St. Louis, Warren Green, 1970; DeLong MR: Brain Res 40:127-135, 1972) |
Figure 16-16 shows the timing of the discharges in neurons of the thalamic nucleus ventralis lateralis, the motor cortex, dentate nucleus, nucleus interpositus, and cerebellar cortex related to the time of onset of muscle activity (lower histogram) and the time of movement of the wrist. On the abscissa is plotted time before (negative numbers) and after (positive numbers) a movement beginning at time zero. The values on the ordinate are the number of changes in firing rate (either increases or decreases) that occurred in relation to that movement(4). Thus, the first changes in thalamic neuron discharges were seen slightly more than 200 msec before the movement. Clearly, neurons at each recording site change their discharges before the movement begins, but the earliest changes are seen in the motor cortex and dentate nucleus. The arrow in each histogram plots the median time of the first change in activity. This indicator of central tendency puts the first discharge in the thalamus, then later in the dentate nucleus, motor cortex, interpositus nucleus, and Purkinje cells. Neurons in the red nucleus (not shown) begin discharging between interpositus and Purkinje neurons. These data do not really allow one to say where a movement begins; neurons everywhere discharge before, during, and after the movement.
One should always keep in mind that a movement of any part of the body involves contractions in muscles other than those intrinsic to that part. Thus, movement of the wrist involves more than just flexors and extensors of the wrist. Figure 16-17 shows some of the muscles that contract when a monkey pushes a lever away from himself or pulls it toward himself. With a rapid pull, the arm extensors contract first about 90 msec before the lever moves (times before the movement are expressed as negative values). This is followed by contractions in the dorsoepitrochlear muscle at 75 msec and so on. Muscles that actually abduct the arm, the latissimus and biceps, do not begin contracting until 75 and 30 msec before the movement. With a rapid push movement, the sequence begins earlier, at about 170 msec before the movement, with contractions of the deep thoracic muscles and other muscles that do not move the arm. In fact, the muscles that extend the forearm, pushing the lever away, do not begin to contract until 90 msec (deltoid) and 70 msec (triceps muscles) before the movement. It cannot be ascertained to which muscle contractions in the sequence a given neuronal discharge may be related. Perhaps part of the variability in histograms of Figure 16-16 may be due to this involvement of many muscles in even the simplest movements.
| The function of supraspinal control of movement |
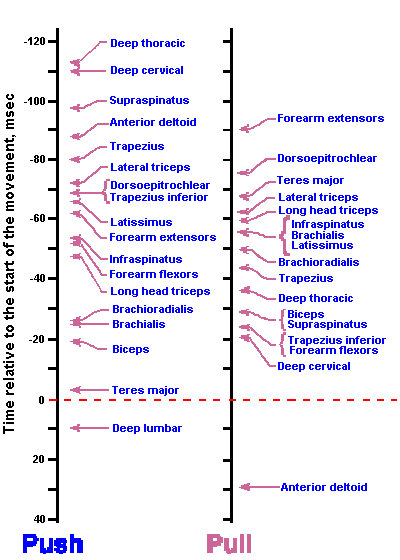 |
Fig. 16-17. Time of contraction of various muscles during a push-pull movement by a monkey. The movement (push, left; pull right) began at time zero; negative time is before movement; positive is after. (DeLong MR, Strick PL: Brain Res 71:327-335, 1974) |
If much of motor control and muscular coordination takes place at the spinal cord level, why have all this other machinery involved in movement? The frog, after all, moves perfectly well (for a frog) without a lot of brain. The answer lies in the smoothness, range, complexity, and plasticity of movements that are possible with these added controls. It is clear that a frog catches flies without a pyramidal tract, and he escapes predators pretty well without large basal ganglia or large cerebellar hemispheres, but with these items and a few others his repertoire of behaviors is nearly exhausted. The basic neural circuitry of the frog fly-catching apparatus is schematized in Figure 16-18A. The frog sits in some environment, E, that contains a flying insect, S; the insect is detected by an exteroceptor, R, activating an automatic, triggered network, TN, resulting in activation of the motor apparatus, M, for fly-catching behavior. The whole event is played out rapidly, but without any elements of prediction. If the nervous system of the frog were "smart," it would soon discover that there are places where flies are likely to be and places where they are not likely to be. Actually, frogs are able to do this, at least to a limited extent. If frogs could make extensive use of this information, the chances of successful flycatching and therefore survival would increase; they would, in short, become better fly traps.
Figure 16-18B is schematic of how such a mechanism might work. Let us say E' is an environment in which flies are likely to be. The frog's exteroceptors sense the presence of E' even though S is not present. This information is transmitted to his cerebral cortex (this is a very unusual frog), which increases the excitability of the exteroceptor network (arrow from cerebral cortex to R), the excitability of the triggered-network (arrow from cerebral cortex to TN), or the motoneurons producing the movements (arrow from cerebral cortex to M). Now everything is ready: S appears in E, and the fly-catching behavior can occur even more rapidly, increasing the chances of success. Not only that, but the information about the whole event, including the quality of the environment E', can be saved for future reference. This ability to predict the need for action and to change sensory function as well as motor function in prewired, stereotyped networks is probably what the cerebral cortical influences give the organism that has them. The cortex is incapable of acting on its own, but can call into play other centers to help in modifying the excitability not only of distal muscles, but also proximal, postural muscles. This makes some sense in light of the evolution of the nervous system, as discussed in Chapter I.
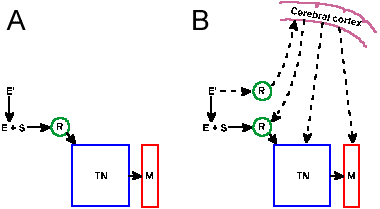 |
Fig. 16-18. A. Basic fly-detecting and catching circuitry of a frog. B. Basic fly-catching circuitry of a "smarter" frog and perhaps the schema for motor organization in mammals. R=exteroceptors and related sensory circuits; E and E'=environments; S=trigger stimulus, in this case the fly; TN=the automatic triggered network whose activity results in the behavior; and M=the motor apparatus that produces the behavior. (Towe AL: Neurosci Res Prog Bull 9:42-48, 1971) |
| Summary |
The basic framework around which movements are organized is the reflex framework of the spinal cord. Many of the basic movements are programmed at that level and only regulated, corrected or directed from supraspinal structures. Transection of the spinal cord produces spinal shock. Recovery involves reappearance of flexion reflexes and later extensor reflexes, with periods of spasticity involving each. The amount of regulation of autonomic functions, body temperature, and endocrine functions remaining, depends upon the level of the cord section. Interruption of the higher neuraxis produces spasticity and, in general, as the interruption occurs at higher levels the spasticity is less. Postural and autonomic functions are more likely to be intact with lesions above the hypothalamic level. Stimulation of brain-stem structures often leads to the occurrence of complex motor patterns, involving sequences of movements. Such patterns are seldom evoked by electrical stimulation elsewhere in the CNS; rather, simple movements of body parts or even contraction of single muscles results. The cerebellum is involved in coordination of muscular activity; deficits in coordination, synergy and timing appear with cerebellar disease and damage to the deep cerebellar nuclei. Less severe, if any, trauma occurs with damage to the cerebellar hemispheres. It is possible that the vermis corrects and modifies movements that have occurred, that the intermediate lobe corrects and modifies movements as they occur, and the lateral lobe participates in planning movements. The function of the basal ganglia is a mystery, but diseases of the various structures result in dyskinesias, postural fixations, and, in some cases, tremor. Movements can be evoked by stimulation of the cerebral cortex. The site of the movement depends upon the site of stimulation. Eye movements are elicited from the frontal eye fields, head movements from a region just medial to them. The pyramidal tract has as many sensory termination sites as motor, and the majority of the fibers terminate in the brain stem, not in the spinal cord. The EPSPs in alpha-motoneurons, that result from pyramidal tract fiber activity, sum in a nonalgebraic fashion, called facilitation. The pyramidal tract may be telling the muscle when to start contracting. Pyramid section leads to paresis, flaccidity, hyporeflexia, and the Babinski sign, whereas cortical damage usually leads to paralysis, spasticity, hyperreflexia, the Babinski sign, and clonus. The latter group of symptoms are those of upper motoneuron disease. Lower motoneuron disease is characterized by paralysis, flaccidity, areflexia, fasciculations, and atrophy. If upper and lower motoneuron symptoms occur together in the same muscle only the lower motoneuron symptoms are seen. The stepping cycle is generated by intrinsic spinal cord mechanisms that can be triggered or modified by sensory and descending influences. The stepping cycle works by modifying reflex activities. Some cells in virtually all supraspinal motor centers discharge before movements begin, but it is difficult to say to what aspect of the movement the discharges may be related.
| Suggested Reading |
- Allen GI, Tsukahara N: Cerebrocerebellar communication systems. Physiol Rev 54:957-1006, 1974.
- Bucy PC: Is there a pyramidal tract. Brain 80:376-392, 1957.
- DeLong MR, Strick PL: Relation of basal ganglia, cerebellum, and motor cortex units to ramp and ballistic limb movements. Brain Res 71:327-336, 1974.
- Dum R, Strick PL: The corticospinal system: a structural framwork for the central control of movement. In: Rowell LB, Sheperd JT (eds) Handbook of Physiology. Section 12, Exercise: Regulation and Integration of Multiple Systems. Oxford: Oxford Univ. Press, 1996.
- Evarts EV: Brain mechanisms of movement. Sci Amer 241:164-179, 1979.
- Glickman SE, Schiff BB: A biological theory of reinforcement. Physiol Rev 74:81-109, 1967.
- Jeannerod M: The Cognitive Neuroscience of Action. Cambridge, MA: Oxford, 1997.
- Lawrence DG, Kuypers HGJM: The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91:1-14, 1968.
- Lawrence DG, Kuypers HGJM: The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 91:15-36, 1968.
- Orlovsky GN, Shik ML: Control of locomotion: A neurophysiological analysis of the cat locomotor system. In Porter R [ed]: International Review of Physiology. Vol. 10, Neurophysiology II. Baltimore, University Press, 1976.
- Picard N, Strick PL: Motor areas of the medial wall: a review of their location and functional activation. Cerebral Cortex 6:342-353, 1996.
- Porter R: Functions of the mammalian cerebral cortex in movements. In Kerkut GA, Phillis JW [ed]: Progress in Neurobiology. Oxford, Pergamon Press, 1973.
- Rothwell J: Control of Human Voluntary Movement, 2nd Ed. London: Chapman & Hall, 1994.
- Ruch TC: Transection of the spinal cord. In Ruch TC, Patton HD [ed]: Physiology and Biophysics, I. The Brain and Neural Function, 20th ed. Philadelphia, WB Saunders, 1979.
- Ruch TC: Brain stem control of posture and orientation in space. In Ruch TC, Patton HD [ed]: Physiology and Biophysics, I. The Brain and Neural Function, 20th ed. Philadelphia, WB Saunders, 1979.
- Towe AL: Untitled quoted contribution in: Central control of movement. Neurosci Res Prog Bull 9:42-48, 1971.
- Towe AL: Motor cortex and the pyramidal system. In Maser JD [ed]: Efferent Organization and Integration of Behavior. New York, Academic Press, 1973.
- Towe AL: Relative numbers of pyramidal tract neurons in mammals of different sizes. Brain Behav Evol 7:1-17, 1973.
Footnotes:
1. It is customary to write parkinsonism with a lower case p, but Parkinson's is written with an upper case P.
2. I've lumped here also a region in the banks of the cingulate gyrus, the cingulate motor area.
3. It is not meant to be implied that a single fiber ends in all of these nuclei; quite the contrary is true. Possible terminations in the cranial nerve nuclei in man are omitted.
4. Cortical stimulus strength was adjusted to give a single EPSP the same size as the group I EPSP in A.
5. In this figure, the frequency of discharge is indicated on the ordinates.
6. For a neuron to be involved in initiating movement, it must change its behavior before the movement occurs. This is the minimum requirement.
[TOC] [Chapter 17] [Glossary] [Index] [Abbreviations]