
 Chapter 4b
Chapter 4b 
SENSORY RECEPTORS II
I n this chapter, we will delve more deeply into the mechanisms of transduction in sensory receptors. First, recall that sensory receptors have 3 functional regions: the receptor region, which contains stimulus-gated channels; the spike-generating region, which contains voltage-gated channels; and the conducting region, which contains voltage-gated channels. The receptor region is the one exposed to the external stimulus. This region contains no voltage-gated channels and is therefore incapable of generating action potentials. The spike-generating region is the place where the stimulus energy is converted to action potentials. There must be voltage-gated channels here, else no spikes would be possible. The conducting region is really just an axon. It must also contain voltage-gated channels or no action potentials would be possible.
The receptor region hypopolarizes when the stimuli open special stimulus-gated channels. These channels are nonspecific, but cations (particularly sodium) predominate and the current is net inward. That inward current is coupled to an outward current (by Kirchhoff's law). This outward current flows in many parts of the membrane, amongst them the spike-generating region. Because the outward current is flowing through resting membrane, it hypopolarizes it. The hypopolarization opens voltage-gated channels in the spike-generating region, the cell is further hypopolarized. If the hypopolarization is great enough (crosses the critical firing level), an action potential results. This action potential is then conducted through the conducting region.
Let's step backward in the process and examine how the stimulus-gated channels are actually opened in a number of different receptors. We will begin this discussion with the muscle spindle receptor, a tiny sensory body located within the body of skeletal muscles whose function is tell the CNS what the length of the muscle is. Having finished with the muscle spindle, we will consider what happens in hair cells like those in the auditory and vestibular apparatuses. Next to last we will consider two chemical sensors, those for gustation and olfaction, and finally, we will take up the receptors in the eye. These last appear to be a bit anomalous, but as we shall see they may not be.
Muscle Spindle Receptors
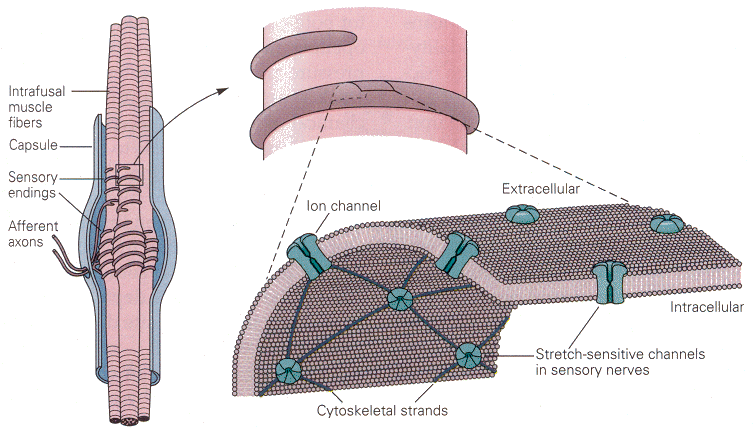
The muscle spindle lies within skeletal muscles. It is arranged in parallel with the extrafusal fibers of the muscle, those that actually do the shortening. In this position, the receptors are optimally placed to sense the length of the muscle and the rate of change of length. The receptor cells themselves (themselves sensory neurons) are actually associated with small striated muscle fibers within the spindle. (You may read more about the structure of muscle receptors in Chapter 11.) On the surface of the sensory neurons are located the stimulus-gated channels. These channels are interconnected with each other and other elements of the cytoskeleton by cytoskeletal strands of spectrin. The channels in question are opened by stretching the membrane; probably the spectrin strands “pull” the channel open when the membrane is stretched. So, mechanical deformation of the membrane opens these cation-selective channels, and there is an influx of sodium and/or calcium, which hypopolarizes the membrane (the generator potential). If large enough this hypopolarization will lead to spikes. The presumed physical arrangement of the channels and spectrin strands is shown in Fig. 1. It is not difficult to see how this arrangement might lead to receptor activation and spike generation.
|
| Figure 4b-1. Left: The structure of the muscle spindle, showing the sensory neurons entering the spindle capsule and wrapping around the small intrafusal muscle fibers. Right (above): A single sensory axon wraps around the muscle fiber. Right (below): A section of the axon membrane showing the ion channels interconnected by the cytoskeletal strands of spectrin. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
The muscle spindle receptors are used by the brain to determine the lengths of the various skeletal muscles and to derive from the lengths the angles of the joints. Clearly, as a muscle changes length, the tension in the muscle changes, and as the tension in the muscle increases the receptor structures discussed above will be deformed. In this case, the transduction is a change from mechanical energy to the electrical energy of spikes.
|
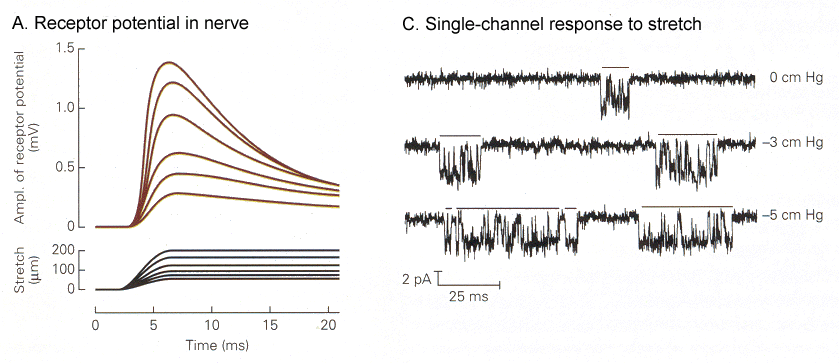
| Figure 4b-2. A. Graded receptor potentials (upper traces) in response to graded amounts of stretch of the spindle (lower traces). B. Increases in the amount of time the ion channel spends open (downward deflection) in response to increasing amounts of stretch (top-to-bottom). Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
In Fig. 2 you can see the receptor potentials elicited by different amounts of stretch of the muscle. The graded quality of the receptor potential is clearly seen on the left. On the right are shown traces of membrane currents made by “patch-clamp”(1) recording. Horizontal bars are placed over the periods of time when the channel is open. A downward deflection here shows an inward current. Note that the channels open only briefly, then close. Activation of the channel causes it to open more frequently and to stay open longer.
|
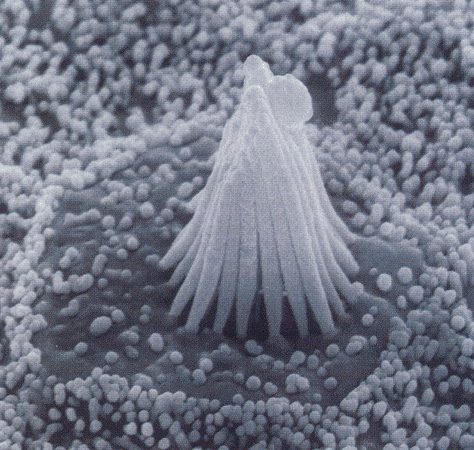
| Figure 4b-3. Scanning electron micrograph of the stereocilia and kinocilium of a hair cell. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
Transduction in Hair Cells
|
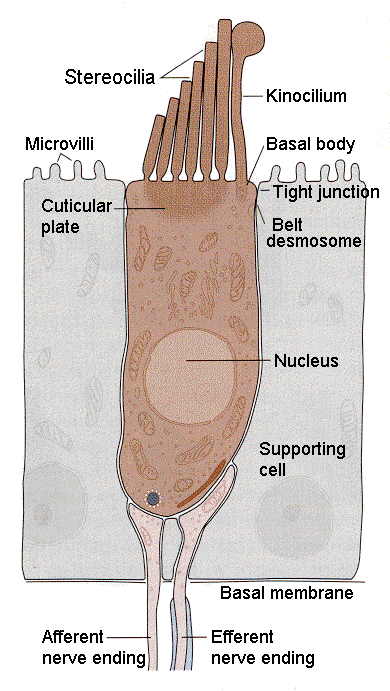
| Figure 4b-4. Schematic diagram of a hair cell showing the relationship of the cilia to the kinocilium and cell body as well as the innervation at the bottom of the cell. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
Hair cells are found in the auditory and vestibular structures of the ear. They are columnar or flask-shaped and have an array of stereocilia at the apical ends. In the vestibular system there is one true cilium (the kinocilium) at one flank of the array of stereocilia. The position of the kinocilium “polarizes” the cell. Fig. 3 shows the arrangement of cilia and kinocilium in a hair cell in the vestibular system. The kinocilia of the hair cells in the auditory system degenerate and so the cells are not effectively polarized. The cilia are bathed in potassium-rich endolymph, a situation different from what we normally encounter. Normally, the extracellular fluid is poor in potassium; here it is rich. The hair cells are form synaptic connections from neurons at the basal ends (the end opposite the stereocilia); these connections can be either afferent (sensory) or efferent (activity coming from the CNS, presumably to exert control of sensory functions). These can be seen in Fig. 4.
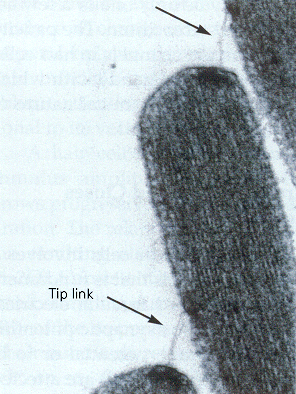
|
| Figure 4b-5. Consecutive tips of stereocilia are shown along with the tip links that bind them together and pull open the ion channels. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
The stereocilia are connected together by a filamentous process, the tip link, which passes obliquely between the distal end of one stereocilium and the side of the longest adjacent one. The tip link is clearly visible in Fig. 5. The current concept is that the tip link connects at one end to an ion channel,
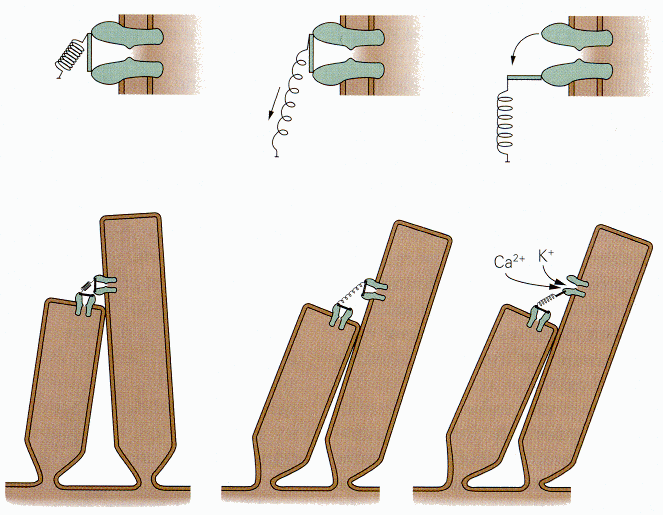
|
| Figure 4b-6. Two adjacent stereocilia with tip link joining two ion channels (probably there is only one). The upper detailed drawing show how the link is stretch by deflecting the cilia and how the channel is opened. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
According to this model, a stimulus that displaces the stereocilia toward the kinocilium (or in the direction where the kinocilium would be in the auditory system), elongates the tip link that pulls on the mechanical gate and opens it. (You may read more about the mechanics of hair displacement in Chapters 8 and 9.) The channel then admits potassium ions; their entry hypopolarizes the hair cell. A schematic diagram of the way this is thought to occur is shown in Fig. 6. Conversely, when the stereocilia are displaced away from the kinocilium, the tension on the tip link is reduced (perhaps it shortens) reducing the pull on the mechanical gate and closing it. Potassium no longer enters the cell, and it repolarizes.
The cation-selective channels in hair cells are blocked by aminoglycoside antibiotics, e.g., streptomycin. Higher doses have toxic effects on hair cells, damaging the stereocilial bundles and eventually killing the cells.
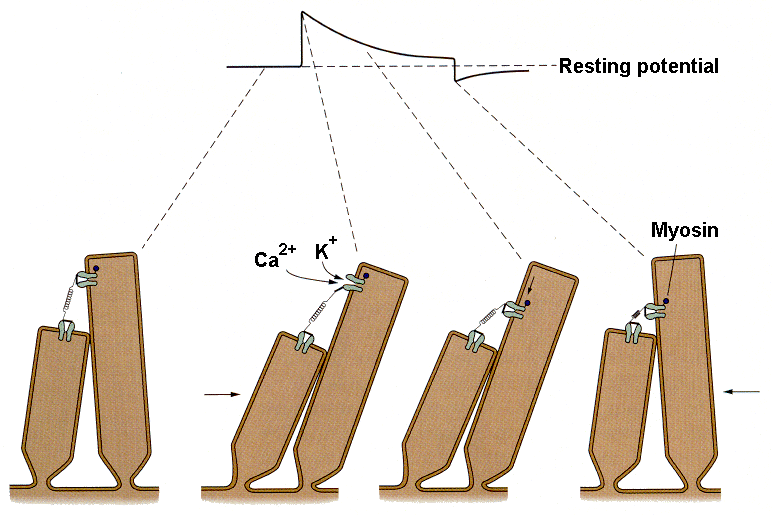
|
| Figure 4b-7. Schematic that shows movement of the stereocilia, the opening of the ion channels and the downward translation of the channel during adaptation. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
With protracted hair cell bundle displacement, the amplitude of the generator potential gradually decreases, i.e., there is adaptation. The rate and extent of the adaptation is known to increase with increasing calcium concentration in the endolymph. This process is thought to occur by the mechanism illustrated in Fig. 7. It appears that the structure anchoring the tip like at its upper end actually moves toward the base of the stereocilium. Current thought is that myosin, the same substance involved in contraction of skeletal muscle, is activated by calcium ions. The calcium ions enter the cell through the same channel that admits the potassium ions that hypopolarize the cell. Calcium, through its interaction with calmodulin and thence with myosin, causes the channel to migrate toward the base of the hair cell, thereby reducing the tension on the tip link and allowing the channel to close. Closing the channel reduces the inward current and allows the cell to repolarize, i.e., to adapt.
For hair receptors the transduction involves a conversion of mechanical energy to the electrical energy of spikes. In the case of auditory receptors, the mechanical energy occurs in the form of alternating compressions and rarefactions of some medium, usually air. In the case of vestibular receptors, the mechanical energy occurs in the form of displacements of hairs due to the force of gravity or to inertia.
Olfactory Receptors
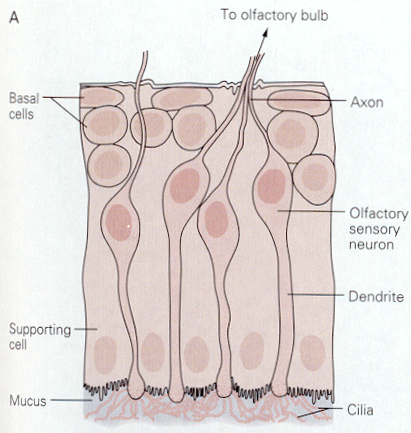
|
| Figure 4b-8. Four olfactory sen-sory neurons are shown with their cilia projecting into the mucus. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
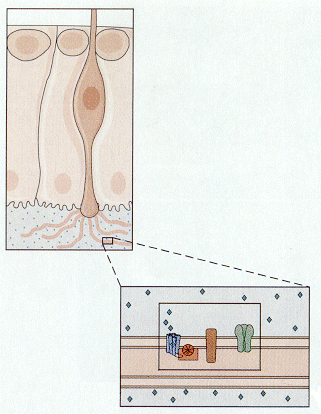
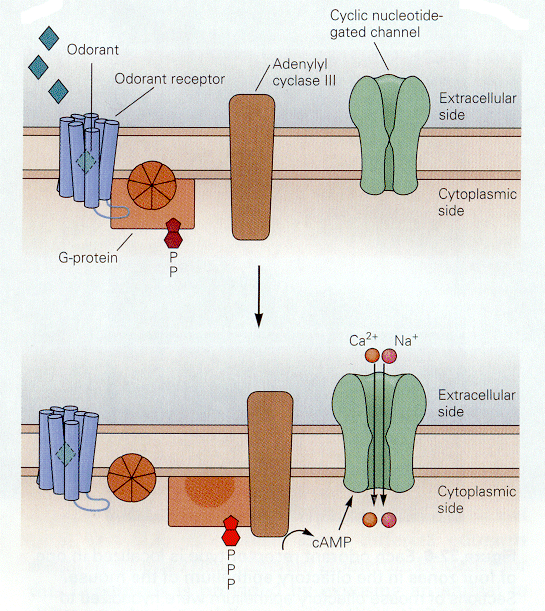
Things that we smell are varied. Attempts to reduce the number of smelled qualities to a few categories have been largely unsuccessful in providing only a few categories. All things smelled are aerosolizable and water soluble because they must reach and dissolve in the mucus that covers the olfactory receptors. Unlike the hair cell receptors, the olfactory receptors are themselves sensory neurons. They have cilia that project into the mucus surrounding the olfactory epithelium as shown in Fig. 8. It is on these cilia that the odorant receptors are thought to be located. Each olfactory neuron expresses only one type of odorant receptor. Binding of the odorants to receptors activates a G protein, which in turn activates adenlyl cyclase to produce cAMP. It is cAMP that activates a cyclic nucleotide-gated cationic channel. When the channel is opened, sodium and calcium enter the cell, hypopolarizing it. The location of the receptors and second messenger cascades and the sequence of events in channel opening are shown schematically in Fig. 9 and 10.
|
| Figure 4b-9. Inset shows an enlargement of a portion of the membrane of a cilium showing the location of the receptor and the G protein system. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
|
| Figure 4b-10. The G protein system in the resting condition (above) and the active condition (below). In the active condition, an ion channel opens admitting cations. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |

Gustatory Receptors
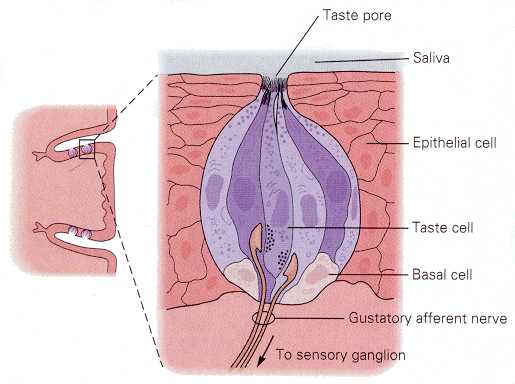
Gustatory receptors, located on the tongue and nearby, are specialized to detect things that are sweet, sour, salty or bitter. There are many other qualities of foods that we associate verbally with taste; all but these four are actually associated with olfactory receptors. Taste buds (groups of gustatory receptors) are comprised of receptors arranged like segments of an orange (Fig. 11). Each receptor has microvilli on its apical end. The microvilli project through the surface of the tongue in taste pores. Like the hair-cell receptors, the gustatory receptors are not themselves nerve cells, but they receive innervation from nerve cells near their bases, again much like hair cells. The arrangement of receptors and nerve cells in a taste bud is shown in Fig. 12.
|
| Figure 4b-11. A group of taste receptors supplying their microvilli to the taste pore. Note the innervation of the taste receptors by nerve cells in the center of the figure. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
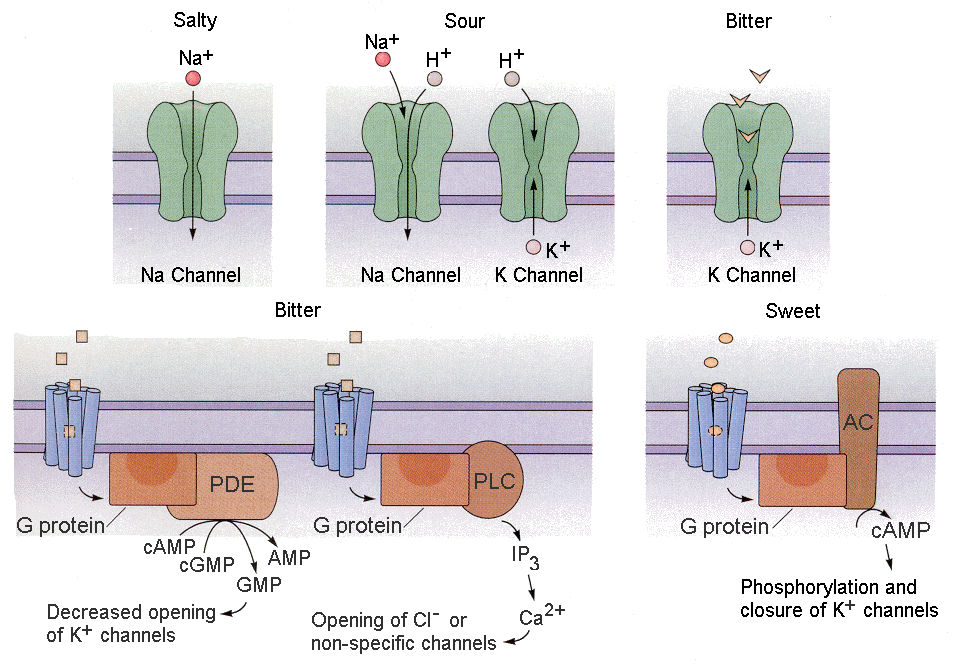
Tastants (things we taste) produce receptor potentials in different ways.
1. Some tastants simply enter the receptor cell through channels as ions. For example, salty substances often contain sodium ions. These sodium ions can simply enter the receptor cell through sodium or cationic channels. Sour substances are acidic. The hydrogen ions can enter cells through cationic channels.
|
| Figure 4b-12. Actions of salty, sour, bitter and sweet substances on their respective receptor systems as described in the text. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
2. Other tastants can compete for use of potassium channels, thereby reducing outward potassium currents (this would result in hypopolarization). For example, bitter substances like quinine can
block the potassium channels, leading to hypopolarization.
3. Still other tastants work through second messengers to close potassium channels, reducing potassium current. Both bitter and sweet substances act in this way.
4. A final group of tastants act through second messengers to open chloride or non-specific ion channels.
Visual Receptors
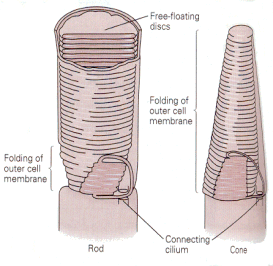
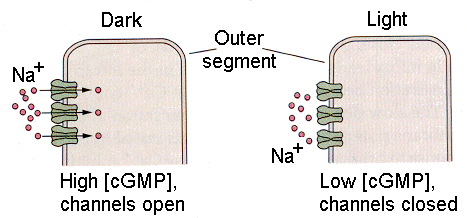
Visual receptors must convert the energy in light to action potentials, at least indirectly. The visual receptors, the rods and cones, do not support action potentials. Their membrane potentials do change in response to light stimulation, but their membranes contain no voltage-gated channels and therefore they cannot produce spikes. There are two kinds of visual receptors–the rods and the cones, so named because of their characteristic shapes. Both types of receptors have an inner segment, consisting of the cell body and efferent process, and an outer segment, connected to the inner segment by a cilium and consisting mainly of layers of folded membrane (Fig. 13). The folded membrane of the external segments contains the visual pigment, a substance that absorbs photons and changes its conformation. All the rods contain the pigment, rhodopsin. The cones contain one of three different pigments. The outer segment membrane contains cGMP-gated sodium channels, which are open in the dark. That means that there is a continuous entry of sodium ions into the cell in the dark, the dark current, that hypopolarizes the membrane of the receptor. Stimulation of the receptors by light turns off the dark current allowing the membrane to repolarize, usually referred to as an hyperpolarization (relative to the state that exists in the dark).
|
| Figure 4b-13. Schematic of the outer segments of a rod (left) and a cone (right) showing the folding of the membranes. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. |
|
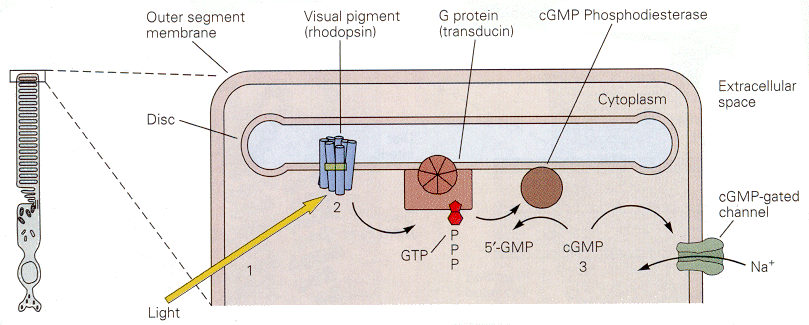
| Figure 4b-14. The cascade of events initiated by light in a photoreceptor. The result of the cascade is to close a cGMP-gated sodium channel, reducing the inward current and hyperpolarizing the membrane. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
The cascade of events that leads from the photon to the cessation of the dark current is thought to involve a G protein. This is shown schematically in Fig. 14. Specifically, the conformational change in the photopigment activates a G protein, which leads to activation of a cGMP phosphodiesterase. The resulting conversion of cGMP to 5'-GMP reduces the amount of cGMP in the cell. Sodium channels gated by cGMP will then close, reducing the inward
|
| Figure 4b-15. Outer segment of a photoreceptor showing the sodium channels open in the dark (left) and closed in the light (right). Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
|
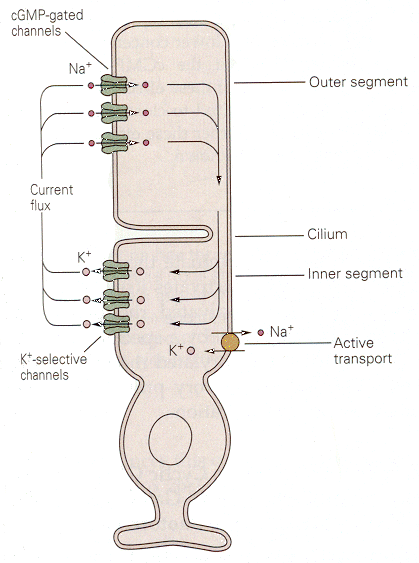
| Figure 4b-16. Dark currents flowing between outer and inner segments of a photoreceptor. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. Click to enlarge picture. |
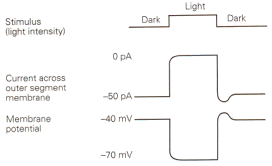
|
| Figure 4b-17. Membrane current and voltage observed in a photoreceptor in the dark and the light. Inward currents are indicated by downward deflections of the current trace. Source: Kandel, Schwartz & Jessell, Principles of Neural Science, 4th Edn. |
We have seen that generator or receptor potentials in all types of receptors except those in the eye are hypopolarizing. A generator potential must hypopolarize because it is the hypopolarization that leads to the action potential. Why then are receptor potentials in the eye of an hyperpolarizing nature? Why are they different? Perhaps it is because these potentials are not generator potentials–they don’t lead directly to action potentials. However, consider the possibility that we have misidentified the stimulus for the eye. We like to think of the stimulus as light, the lack of a stimulus as dark. It is possible that the real stimulus is dark.
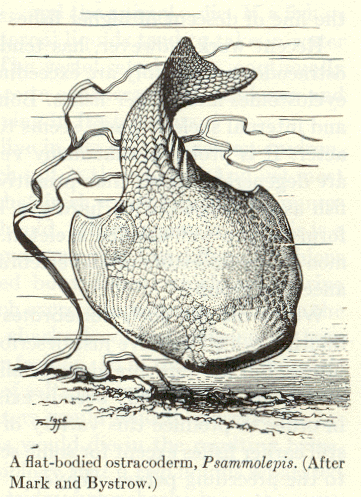
|
| Figure 4b-18. An ostracoderm from A.S. Romer, The Vertebrate Story, Chicago: University of Chicago Press, 1959, Page 41. |
Consider the plight of some ancient ancestors of vertebrates, the ostracoderms (Fig. 18). These jawless fish-like creatures probably swam around their environments with their mouths open, filtering the water near the floor of lakes or rivers for edible morsels. Clearly the ostracoderms were not predators. Now, the ostracoderms were probably a favorite food of an invertebrate predator, the eurypterids or water scorpions (Fig. 19). Clearly, the eurypterids overmatched the ostracoderms based on their much larger size. The only defense for the ostracoderm was its bony outer covering, hence the name. Speculate with me for a moment. Suppose that these mainly defenseless fish-like creatures developed a type of cell (a receptor) that contained a photopigment and was capable of secreting a substance (let’s call it a hormone) that could cause the animal to begin swimming. Further suppose that the substance was released from the cells when the receptor was shaded–it’s a shadow detector. During the Ordovician period when these animals lived there were few large land plants to cast shadows across the water. Because of this, the major source of shadows would have been other creatures, among them the eurypterids.
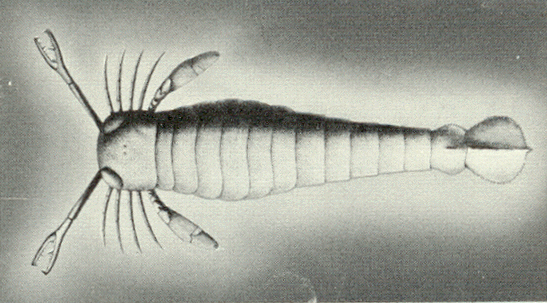
|
| Figure 4b-19. A eurypterid from A.S. Romer, The Vertebrate Story, Chicago: University of Chicago Press, 1959, Page 8. |
An approaching predator would cast a shadow upon the primitive photoreceptor, the cell would release its hormone and the ostracoderm would swim away to safety. Clearly, this would convey a survival advantage on the animal who possessed such an ability. Now suppose that an ostracoderm possessed such a photoreceptor but with a longish process that brought the release point for the hormone closer to the target muscles. That animal would have an advantage over one without such a process. According to Darwinian logic, that animal would be selected for survival. The longer the process, the more likely the survival. Gradually, the cellular arrangement would come to look less like an element of an hormonal system and more like an element of a nervous system. If this scenario has any accuracy at all, then the first photoreceptors may well have been shadow detectors for which the stimulus was a shadow, i.e., darkness. By this logic, then our visual receptors may well have developed from such shadow detectors. During the process of evolution, we came to use these receptors in a different way, emphasizing the presence of light more than its absence. In light of this argument, it is clear that the actual stimulus for the photoreceptor, in paleontologic terms, may not be light but dark.
Summary
Transduction is the process of converting stimulus energy to action potentials. Different receptors do this job in different ways. Some use already open channels through which the stimulus passes (gustation). Some open channels directly by mechanical linkages (muscle spindles, hair cells). Others block or compete for potassium channels (gustation), whereas still others activate second messenger systems. These second messenger systems can open or close cyclic nucleotide-gated, cationic channels, or open chloride channels, or close potassium channels. Visual receptors appear to anomalous in that their receptor potentials in response to illumination are hyperpolarizing. However, these receptor potentials do not lead to action potentials. They do not appear to be unusual if you think of the stimulus as being dark not light.

Footnote:
1. A patch clamp is used to measure small membrane currents such as those that flow through a single channel. A polished micropipette electrode is brought against the membrane of a cell, and a small amount of suction is applied to the pipette. When all goes well the pipette will “seal” to the membrane offering an extremely high resistance for current to flow between the pipette and the membrane. That means that currents will flow from the membrane into the pipette. Without this technique these small currents will be lost in the noise that comes from this leak around the pipette.

[TOC] [Chapter 5] [Glossary] [Index] [Abbreviations]