Chapter 6
Chapter 6 
SOMESTHESIA - CENTRAL MECHANISMS
In this chapter, we will examine the pathways that somatic activity can take in the central nervous system, and then we will consider the mechanisms by which different sensations can occur.
| Somatosensory pathways |
Once the primary afferent fibers leave the skin, they group together into larger and larger bundles in the cutaneous nerves. At some point in their course they are joined by muscle nerves, containing sensory and motor fibers of the muscles. Nerves that innervate the skin are termed cutaneous nerves, and those that contain both skin and muscle fibers are termed mixed nerves. The sciatic nerve is an example of a mixed nerve. Nerve fibers that carry impulses to the CNS are called afferent; those that carry them from the CNS are efferent. The afferent and efferent fibers are separate from each other at the skin or muscle, but they are usually mixed together along most of the course of the nerve on the periphery. Near the spinal cord, the afferent and efferent fibers separate again, the efferent fibers going to the ventral (or anterior) roots and the afferent fibers, after passing their cell bodies in the dorsal root ganglia, going through the dorsal (or posterior) roots. This separation of afferent fibers in the dorsal root and efferent fibers in the ventral root is the so-called Bell-Magendie Law. Lately this concept of a rigid separation of dorsal, sensory and ventral, motor fibers has been challenged by findings that, in the cat, up to 30% of the ventral root fibers are sensory. This probably would not have surprised Magendie, who originally stated that most fibers in the ventral roots were motor.
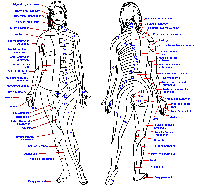
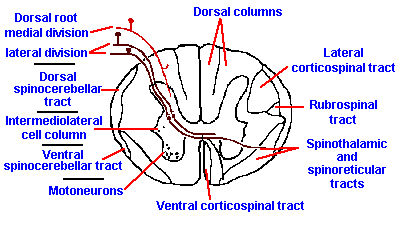 |
Fig. 6-1. A cross section through the cervical spinal cord showing the entry of the two divisinons of the dorsal roots and the routes that fibers take into the major ascending tracts. (Crosby EC, Humphrey T, Lauer, EW: Correlative Anatomy of the Nervous System. New York, Macmillan, 1962) |
The dorsal roots are often divided into two parts, a lateral division consisting mainly of unmyelinated fibers and a medial division consisting mainly of larger, myelinated fibers (see Fig. 6-1). Upon entering the spinal cord, some of the afferent fibers bifurcate, with one collateral descending toward the caudal regions of the cord and the other ascending toward the rostral regions. Both myelinated and unmyelinated fibers branch extensively and have terminals near the dorsal root entry zone. The collaterals of the unmyelinated fibers go only a short distance, one or two segments, before terminating in the dorsal horn, but a fiber may put down several collateral branches that terminate in many places in the gray matter. Descending branches of the myelinated fibers generally terminate within one segment from their entry; however, many of the ascending branches can travel uninterrupted for long distances in the dorsal white matter, the dorsal columns, even reaching the lower medullary levels where they terminate in the dorsal column nuclei, the nuclei gracilis (lower limb) and cuneatus (upper limb).
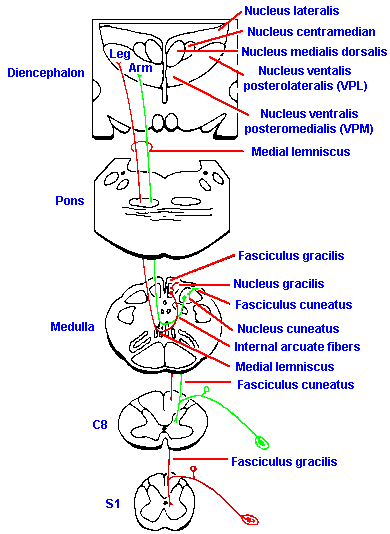 |
Fig. 6-2. The course and major relay points of the dorsal column system. (Crosby EC, Humphrey T, Lauer, EW: Correlative Anatomy of the Nervous System. New York, Macmillan, 1962) |
In the dorsal column nuclei, these fibers form connections with cells that live within the nuclei themselves. As illustrated in Figure 6-2, the axons of these second-order neurons immediately cross over to the other side (decussate) of the brain stem, forming a bundle of fibers, called the medial lemniscus. They are soon joined by fibers of the trigeminal system, serving the face, and also by fibers from the spinothalamic tract.
Those fibers that send a branch into the dorsal columns at the segment of entry also send branches down into the gray matter. There they join the branches of other myelinated fibers and unmyelinated fibers to participate in reflexes and other spinal cord activities. Some of these fibers terminate on cells of the gray matter (second order cells), the axons of which cross in the anterior commissure to the contralateral ventral gray columns at or near the segment of entry and proceed into the ventrolateral white columns. In this position, they ascend the spinal cord as the spinothalamic tract, until they join the fibers of the already decussated medial lemniscus in the brain stem. It is wrong to think of the spinothalamic tract, and perhaps any other central nerve tract, as being a compact bundle of fibers all of which have the same origin, destination and function. In fact, within the region occupied by the spinothalamic tract there are axons of many other ascending and descending tracts, making it virtually impossible to transect spinothalamic fibers and only spinothalamic tract fibers.
Primary afferent fibers from the region of the face in front of the ears group together into the trigeminal nerve and have their cell bodies in the semilunar ganglion. From the semilunar ganglion the fibers enter the brain stem as a part of the fifth cranial nerve. As in the spinal cord, many of the sensory fibers bifurcate immediately upon entering the brain stem, sending an ascending branch to the main sensory nucleus of the trigeminal and a descending branch to the nucleus of the spinal tract of the trigeminal, a structure analogous with the dorsal aspect of the spinal cord gray matter. Though most of the fibers arising from the second order neurons of the trigeminal spinal nuclei decussate in the brain stem and soon join the medial lemniscus, a few do not cross, but join the ipsilateral medial lemniscus.
The fibers of the medial lemniscus, along with those from the trigeminal nuclei and the spinothalamic tract, proceed directly to the ventral part of the thalamus, contralateral to the primary afferent fibers associated with them. Fibers originating in the main sensory nucleus and the rostral parts of the spinal nucleus of the trigeminal terminate in the nucleus ventralis posteromedialis (VPM) of the thalamus, whereas those of the caudal part of the spinal nucleus terminate in the intralaminar nuclei and the medial geniculate, and a few end in VPM. Fibers originating in the dorsal column nuclei and most fibers of the spinothalamic tract terminate in the nucleus ventralis posterolateralis (VPL) of the thalamus. Some spinothalamic fibers terminate outside of VPL, notably in the medial geniculate body and suprageniculate nucleus.
The third-order fibers arising from the thalamic nuclei can then be traced out through the internal capsule to the somatic sensory cerebral cortex. In man, the primary somesthetic cortex is the region of the postcentral gyrus, the region immediately posterior to the central sulcus, from the Sylvian sulcus to a place within the midsagittal sulcus.
There are other pathways that carry somatic information and other areas of the cortex that receive it, but they play a more subtle and less well-understood role in sensation, and consequently there is less we can say about them.
It has been customary to think of the dorsal column-medial lemniscus pathway as the mediator of touch, pressure, vibratory (pallesthesia), and joint position senses. The spinothalamic tract was usually thought to carry information about pain, deep pressure, temperature, and crude touch. The difference between the information in the two pathways was thought to be the sensitivity or threshold (the dorsal columns being more sensitive) and the discriminability of the spatial qualities of the stimulus (the ability to locate the site of stimulus being much greater for the dorsal columns). In the trigeminal system, the caudal part of the spinal nucleus (some people still maintain the whole spinal nucleus) was thought to carry information for pain, temperature and perhaps also crude touch, whereas the main sensory nucleus was thought to relay information from the face about touch, pressure and vibration.
There is an increasing body of evidence that has cast doubt on the dichotomy of function of the systems outlined above. Some of the evidence comes from laboratory experiments. When recordings were made of the discharges of single identified spinothalamic tract cells, it was found that some of them did, in fact, respond to noxious or thermal stimuli, in much the same way as nociceptors or thermoreceptors discussed ln the last chapter. In the monkey, these made up 30% of the fibers studied. Other fibers responded to light touch, pressure, or hair movement, in much the same manner as the mechanoreceptive fibers found in the dorsal columns. These made up 40-60% of those fibers studied. Most spinothalamic tract fibers (60%) responded to both light tactile stimulation and noxious stimulation (Trevino DL, Coulter JD, Maunz RA, Willis WD: Localization and functional properties of spinothalamic cells in the monkey. In Bonica JJ (ed): Advances in Neurology, Vol. 4 Pain. New York, Raven Press, 1974).
Conversely, some dorsal column fibers respond to stimulation that is frankly noxious. These fibers are located primarily deep within the columns and are second-order, rather than primary afferent neurons. These second-order neurons make up 10-30% of the dorsal column fibers by some estimates, and are comprised of fibers that respond to light tactile as well as noxious stimulation (77%), noxious stimulation only (6%), and light tactile stimulation (17%) (Angaut-Petit D: Exp Brain Res 22:471-493, 1975). There appears to be some discrepancy between the responsiveness of single fibers and the roles normally attributed to the tracts that contain them. The same sort of mixture of nociceptive and tactile responses has also been found in the main and spinal trigeminal nuclei. Thus, the supposedly complete segregation of submodalities in the different pathways does not hold up to physiological testing. Moreover, at least 3 other major ascending pathways have been shown in animal studies to carry both tactile and nociceptive information.
Finally, cells that could be responsible for signaling the position of a joint, the muscle and perhaps joint receptors, do not form a part of the dorsal column-medial lemniscal system (Clark FJ: J Neurobiol 3:101-110, 1972). The original data suggesting the dorsal columns carry joint position information came from patients with tabes dorsalis, a syphilitic infection which causes degeneration of dorsal column fibers, but tabes dorsalis is a dorsal root disease and more than just dorsal column fibers are affected.
Additional evidence that casts doubt on the dorsal column-spinothalamic dichotomy comes from the clinical literature. Because the spinothalamic tract and the dorsal columns serving one side of the body are on different sides of the spinal cord and because the dorsal columns are on the dorsal aspect and the spinothalamic tract is on the more ventral aspect of the cord, it is possible to transect one without cutting the other(1). In some patients with intractable pain, pain that is not diminished by drug treatment or even by cutting the dorsal roots (dorsal rhizotomy), the spinothalamic tract was cut (spinothalamic tractotomy) at the appropriate level on the side opposite the location of the pain. These patients usually received immediate relief from the pain as predicted, but there were two problems: (1) pain eventually returned in patients that survived longer than 6 months, and (2) there was a reduction in tactile sensitivity (hypesthesia) as well as pain (hypalgesia; note algesia = pain) and temperature sensitivity on the opposite side of the body.
These observations, not often made because tractotomies are seldom performed on patients who are not terminally ill, do not exactly fit the dichotomy of function between the two ascending systems. Likewise, surgical lesions of the dorsal columns in animals may result in little or no permanent tactile loss and little analgesic effect. When the dorsolateral columns are included in the lesion, there is a temporary analgesia (the absence of pain) with gradual recovery of pain sensation. Ventrolateral column lesions (including the spinothalamic tract) are even less effective in producing analgesia. In man, lesions of the dorsal columns produce relief from phantom limb pain, but pain returns in several months. The same is true for various sorts of pain and ventrolateral cordotomy. We will have more to say about this problem later.
On the other hand, damage to half of the spinal cord in man leads to ipsilateral paralysis of voluntary (as opposed to reflex) movements, loss or reduction of touch and joint sensation ipsilaterally, and loss of temperature and pain sensation contralaterally, the Brown-Sequard Syndrome. This is as predicted from the classical notion of dorsal column and spinothalamic tract function, but remember that there are other tracts within half of the spinal cord. At this point, the classical notion of the function of the spinothalamic tract and the dorsal columns is still widely accepted. However, the wise student should expect this notion to be revised before too long and sensory transmission to be attributed to multiple tracts.
| Dermatomes and somatotopic organization |
Fibers serving particular areas of skin gather together to form nerves, and the area served by the different cutaneous nerves can actually be mapped. Such a map is shown in lateral parts of Figure 6-3. Each area is the combination of the receptive fields of all the fibers in that nerve, i.e., it is the receptive field of that nerve. Despite some variation from person to person, the receptive fields of nerves are constant enough in location and extent to be clinically useful.
When a peripheral nerve is cut, the result is an area of complete lack of sensation or anesthesia. The region is called the autonomous zone and corresponds to the area in the central part of a nerve's receptive field. Only a part of the total field is anesthetic, as a natural consequence of the overlap of receptive fields of neighboring peripheral nerves. Surrounding the insentient autonomous zone is an intermediate zone in which there is partial sensation. Sometimes the overlap is so extensive that the loss of a small nerve is hardly noticed.
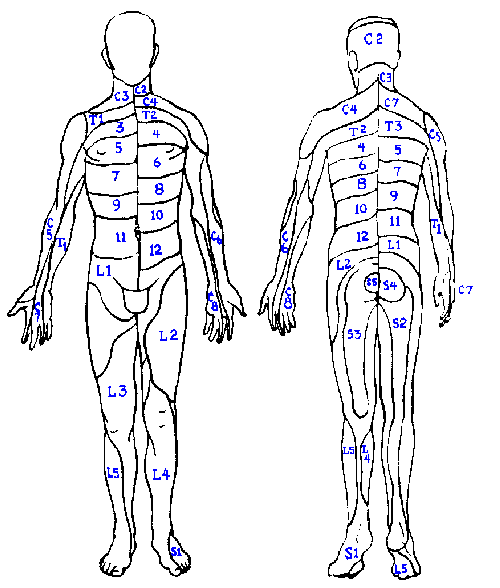 |
Fig. 6-4. Spinal dermatomes plotted alternately on opposite sides of the body to illustrate the entire extent of the dermatomes. This figure should be compared with Fig. 6-3. (Turek SL: Orthopaedics: Principles and Their Application, 3rd ed. Philadelphia, JB Lippincott, 1977) |
The receptive fields of the fibers in a dorsal root, when combined also cover a fixed, distinct area, the dermatome ("slice of skin"). The dermatome of each dorsal root is illustrated in Figure 6-4. Dermatomal organization is a constant feature of the nervous system. Dermatomes overlap in the same way that the receptive fields of both nerves and single fibers do. There is, however, a region in each dermatome that is not overlapped by adjacent dermatomes. In the case of a transected root, one should expect to see hypesthesia in the regions indicated in the central part of Figure 6-3. This sort of map is made by tracing areas of hypalgesia following rupture of intervertebral disks. Note that corresponding areas are smaller than the dermatomes of Figure 6-4. Again, the overlap of adjacent dermatomes may be so great that a deficit is hardly noticed unless two or more dermatomes are denervated! This is especially true of thoracic dermatomes for which there may be little or no nonoverlapped region.
The nerves serving adjacent areas of skin do not necessarily enter through the same or even adjacent dorsal roots. This is especially true on the limbs and results from the pattern of development of the limb buds. For further information, consult a textbook of embryology.
| Knowledge of dermatomes is useful in diagnosis of some neurological as well as non-neurological disorders. |
Knowledge of the receptive field and dermatomal maps is fundamental in examination of any complaint that involves anesthesia, analgesia, hypesthesia or hypalgesia over a portion of the body. For example, a patient with an anesthesia on the posterior side of the leg, running from over the buttocks down to the most lateral toe most likely has a compressed or damaged S1 dorsal root, and a disk problem might be suspected. On the other hand, the patient with an analgesia over the middle of the abdomen, in the general region of the rectus abdominis muscle, extending upward to about the region of the clavicle cannot have damaged a dorsal root or even a group of dorsal roots; the damage must be to the anterior thoracic rami. In the first case, you would look for damage somewhere between the dorsal root ganglion and the spinal cord and, in the second case, somewhere peripheral to the ganglion.
Several diseases involving the dorsal root ganglia are conspicuous because of their dermatomal patterns of symptoms. Tabes dorsalis, a syphilitic infection of the dorsal root ganglia, often exhibits a dermatomal pattern of hypesthesia because the spirochete shows a preference for larger myelinated fibers. Pain and thermal sensations are normal in the affected area. Herpes zoster, a viral infection of the dorsal root ganglia, sometimes called shingles, is characterized by the appearance of a vesicular rash in the dermatome of the affected root.
Knowledge of the dermatomes has other uses besides diagnosing dorsal root damage. In cases of spinal cord transection, the level of section can often be determined by reference to the pattern of sensory loss. The same is true for tumors of the spinal cord that result in a sensory loss. Later we will see that the pattern of motor deficits is equally telling in the neurological exam. Many times visceral pain is felt to be on the surface of the body (so-called referred pain). Often this pain is localized to a dermatome, and knowledge of the dermatomal pattern can lead the alert clinician to the internal organ that is involved.
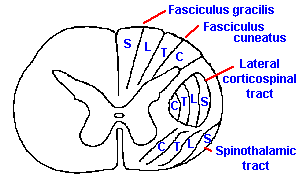 |
Fig. 6-5. The somatotopic organization of the dorsal columns and the spinothalamic tract and the functions classically attributed to each. (Brodal A: Neurological Anatomy, 2nd ed. New York, Oxford Univ. Press, 1969) |
One of the striking features of the organization of the nervous system is that a picture of the body (though distorted) can be mapped onto the various tracts and nuclei of ascending systems all the way to the cerebral cortex. The tracts are formed so that spatial information from the periphery is preserved in the spatial arrangement of the fibers themselves. This type of organization is called a somatotopic representation, referring to the organization by place, "topos," on the body, "soma." The dorsal columns, for example, are formed by the addition of new fibers, at progressively more rostral segments, to the lateral aspect of the columns. This results in an organization such that the dermatomes are laminated, with the sacral segments represented medially and lumbar segments more laterally in the fasciculus gracilis and the thoracic and cervical segments successively more laterally in the fasciculus cuneatus. This organization is illustrated in Figure 6-5.
As a result of this somatotopic organization, it is possible for a spinal cord tumor to disrupt transmission through only the medial fascicles of the dorsal column, producing sensory disturbances in the lower limbs, or through the lateral fascicles, producing sensory disturbances only in the arms. However, it would be extremely unlikely that any lesion of the dorsal columns could produce a sensory disturbance localized to just one dermatome.
The spinothalamic tract likewise appears to be somatotopically organized, but because the fibers are crossed the organization is reversed such that fibers from caudal segments lie laterally, being displaced that way by the progressive addition of fibers to the medial aspect of the tract. (Again, see Figure 6-5). This is presumably why superficial cuts in spinothalamic tractotomies produce immediate analgesia and athermesthesia, i.e., the loss of temperature sensations, only in the contralateral lower limb, with graded, more rostral losses resulting from deeper cuts. These deficits are always contralateral to the damage as opposed to the ipsilateral losses that occur with dorsal column lesions.
It should be mentioned at this point that the somatotopic maps are summary diagrams and should not be taken to indicate that within any marked zone are found only fibers of that type. This is a mistake made frequently. For example, in Figure 6-5, a closed figure surrounds the location of the corticospinal tract; within that figure are found many fibers that are not part of the corticospinal tract. The same warning applies to the somatotopic zones within the dorsal columns or spinothalamic tracts. Within the zone marked for thoracic fibers are found fibers from other levels as well, but the majority appears to be from thoracic segments. This may be one reason why unpredicted effects on sensation are found following controlled tractotomies that do not encroach on the neighboring regions. In any case, these somatotopic maps, whether derived from physiological or anatomical observations, should be treated as statistical statements only.
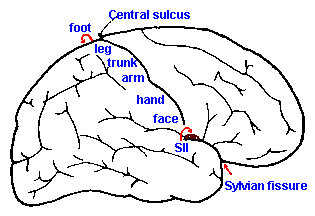 |
Fig. 6-6. The somatotopic organization of the postcentral gyrus of the cerebral cortex and the position of somatosensory area II |
The somatotopic arrangement is preserved at higher levels of the nervous system, though the orientation changes. In the dorsal column nuclei, the arrangement found in the dorsal columns is preserved, with lower limbs represented medially and upper limbs laterally. As they course into the medial lemniscus, the fibers of the gracile nucleus take up a ventral position, and those from the cuneate nucleus a dorsal position. Fibers of the spinothalamic tract join the lemniscus, after it decussates, in such a way that they form a cap on the lemniscus, with the leg represented dorsally and the arm ventrally. Addition of fibers from the trigeminal to the most dorsal aspect results in a representation (from top to bottom) of: face, lower limb, upper limb, trunk and lower limb. In the thalamus, the lower limb fibers terminate laterally and those of the upper limb more medially (the opposite of the spinal cord), with the face represented most medially in VPM. The fibers again cross each other upon leaving the thalamus with the result that the lower limb is represented on the medial aspect of the hemisphere, whereas the representation of the upper limb is more lateral and that of the face most lateral. The resulting organization is illustrated in Figure 6-6. Another representation of the body exists on the cerebral cortex, the so-called somatosensory area II (SII). Its organization is the inverse of that already described for somatosensory area I, namely, face, arm, and leg in that order, going laterally. Area II, however, is not visible on the exposed surface of the human cortex, but is buried in the Sylvian fissure.
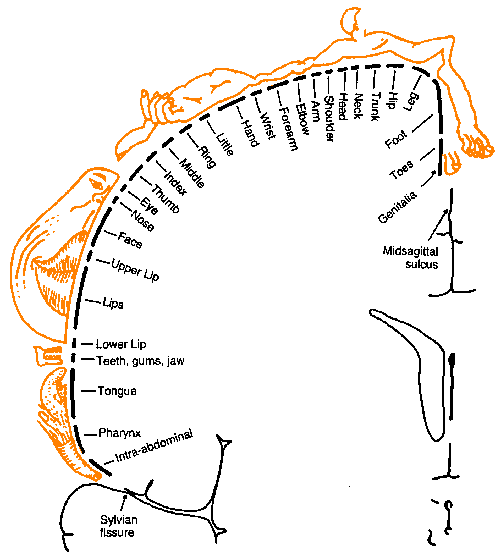 |
Fig. 6-7. A detailed somatotopic map of the postcentral gyrus (Penfield W and Rasmussen T: The Cerebral Cortex of Man. New York, Macmillan, 1969) |
These are rough maps at best. Adjacent areas in skin are represented in adjacent areas of the nervous system. However, it is generally not true (or not known to be true) that adjacent points on the skin have adjacent representations.
This description of the organization of the somatosensory cortex is supported by recordings made directly from the cortex in humans as well as animals. It is possible to open the cranium of an awake human patient under local anesthetics and record from or stimulate the cortex while the patient is awake. This is normally done during surgery to remove a tumor or to treat an epileptic condition. When recordings are made under such conditions, it is usually found that the largest amplitude responses for stimulation of the hand are found on the lateral aspect of the postcentral gyrus, whereas those for stimulation of the leg are on the medial aspects or buried in the midsagittal sulcus. Similarly, if the cortex is stimulated directly, it is possible to elicit sensations referred to a particular point on the body, not to the cortex itself. Even though it is the cortex that was stimulated, the feeling is one of being stimulated on the appropriate body part. The particular point of reference depends upon the site of stimulation in the same orderly manner. A detailed map of the human cortex has been made in this way by Dr. Wilder Penfield, the distinguished neurosurgeon. His map is shown in Figure 6-7. Notice that the foot is represented on the medial aspect of the hemisphere in the midsagittal sulcus with the leg represented on the medial part of the exposed hemisphere, and the trunk, arm and hand more laterally. The face is separate and more lateral still. The amount of tissue devoted to the hand and face is disproportionately large. Some investigators think that this reflects the heightened sensitivity of these parts compared to the trunk and legs, as indicated, for example, by the differences in two-point thresholds.
A caution should be introduced in regard to somatotopic maps like that shown in Figure 6-7. In humans, these maps are obtained in two ways. In the first, various sites on the cortical surface are stimulated, where the patient feels a sensation to be is noted, and a mark is made on a photograph of the exposed cortical surface. Not every point on the surface is stimulated in such an experiment; neither is a sensation experienced at every point of the homunculus in every experiment. Rather, it is assumed that if stimulation at two points on the surface of the brain leads to sensations at two points on the body, then stimulation between the points will lead to a sensation localized to the body surface between the two original points. In other words, it is assumed that an intermediate value principle holds for such maps--a fact not known to be true.
The second mapping method involves recording from the cortex while stimulating the skin. Animals cannot indicate the nature or location of any sensations they have, so this is the only method available in animal experiments. It is assumed that the evoked potential (the combined synaptic responses of all active nerve cells near the recording point) recorded from the cortical surface, is an indicator of the occurrence of a sensation (an assumption that is questionable and probably incorrect). Points on the homunculus are then indicated at places where the evoked potential with largest amplitude occurs; the fact that potentials of smaller amplitude are evoked in wide areas of the cortical surface by the same stimulus is ignored. Activity initiated at a point on the body surface converges upon an area of cortex, not upon a point of cortex as the homunculus is usually taken to imply.
That this method of mapping is grossly reliable is indicated by the rough similarity of maps made in different patients and by the similarity of maps made by stimulating the skin and, in different patients, by stimulating the cortex. However, such maps do vary from individual to individual. Some studies (Woolsey, Erickson, Gilson: J Neurosurg 51: 476-506, 1979) clearly show that in some individuals the foot is represented within the midsagittal sulcus, whereas in others it is represented laterally on the exposed surface. It is also important to remember that figurine maps are composites from a number of patients, each of whom was studied only to a limited extent. Thus, the foot may be studied in one patient, the arm in another, and so forth. If there is variability in the position of the representation, then the amount of tissue devoted to a particular area of skin is likely to be overestimated in composite maps.
It should always be kept in mind that these patients are undergoing surgery for an abnormality, tumor or epileptic focus, in or near the area being studied, and their cortices are not entirely normal. The significance of such maps in understanding cortical organization is also brought into question by the fact that if maps are made using minimum latency of the evoked potential (the time between the stimulus and the beginning of the potential) rather than amplitude as a measure of activity, then a completely different homunculus map is obtained. Who can say whether latency or amplitude is more important?
| Cerebral mechanisms of sensation |
The precise reason for this somatotopic representation in all of the main sensory pathways and nuclei is unknown, though many are of the opinion that it provides the functional and anatomical substrate for coding of spatial localization. Perhaps it does, but perhaps it exists as a mechanical and architectural convenience for a nervous system developing through phylogeny from a simple segmented, metameric pattern.
To see something of the controversy regarding this topic, take a look at JH Kaas. Topographic maps are fundamental to sensory processing. Brain Res. Bull. 44: 107-112, 1997 and RJ Weinberg. Are topographic maps fundamental to sensory processing? Brain Res. Bull. 44: 113-116, 1997.
| Primary somatosensory cortical lesions produce hypesthesia and disabilities in using somatic sensory information to solve problems. |
Many people have the mistaken idea that the cerebral cortex is where the real business of the nervous system is transacted and the rest of the nervous system is just a conduction pathway to it. This is not the case. For instance, removal of the hand area of the postcentral gyrus does not produce anesthesia in the hand. What it does produce is an elevation of the threshold for sensation at the hand, an hypesthesia, and a severe deficit in discriminating the spatial aspects of stimulation, i.e., in using sensory information to solve problems. The patient, following such a lesion, has difficulty in pointing to a site on the skin that has been stimulated (atopognosis); has difficulty recognizing words written on the skin (agraphesthesia); and has difficulty in identifying objects by feeling them (astereognosis). The clinical literature contains some studies that indicate that sensory defects following damage to the primary somesthetic cortex are more severe than claimed above and some that indicate they are less severe. The great variability may be ascribed to a number of factors:
- Naturally occurring lesions are seldom confined to the primary somesthetic cortex; one would expect the nature of the sensory losses to depend upon the total extent and distribution of damage.
- The measures of sensory capacity used in different studies vary widely; results may depend upon the test employed.
- In cases of clinical ablations, the lesions were made in tissue already damaged sufficiently to produce epileptic discharges or already invaded by tumors; the influence of these factors cannot be evaluated. This is especially important when there has been no measure of the sensory capacity of the individual before the surgery.
- What is usually reported is the location and amount of tissue the surgeon removed, but no information is available about what influence the surgery may have had on surrounding tissues. This influence shows up in cases where, after unilateral ablation, deficits are seen bilaterally when the patient is compared to normal people (Corkin S, Milner B, Rasmussen T: Arch Neurol 23:41-58, 1970). Without postmortem examination, there can be no idea how extensive this effect may have been.
- Even when there is postmortem information, it is difficult to determine how much of the damage present was done before, at the time of, or after the surgery.
The minimum effect of a cortical lesion probably is not a good indicator of the function of the tissue because the minimal lesion probably doesn't damage the tissue enough. The maximal effect probably represents the function of too many tissues. Therefore, it seems likely that some middle point is at least a good approximation of the deficits due to removal of this tissue. It really should not surprise us that the cerebral cortex is not required for sensation. Consider how well a frog can sense and act upon stimuli applied to its skin, and it has no cerebral cortex at all.
How can we account for the fact that sensations can be evoked by stimulation of the human somatosensory cortex? Early stimulation experiments resulted in sensations described by the patients as tingling or prickling sensations or a feeling of numbness, and so it was thought not to be possible to evoke "real" sensations, such as touch, by direct stimulation. With improved techniques, such sensations have been evoked, but it is necessary to pass current through the electrodes for about 0.5 sec, a time much longer than that needed simply to excite local neurons. Under these circumstances, identifiable, well-localized sensations have been evoked. Certainly a sensation does not require a cortical neuron to discharge for 500 msec, because for a stimulus to the skin, the stimulus is sensed and a response is executed by a normal person in about 200 msec. One explanation is that the sensation occurs as the result of some event occurring at a distance from the cortical stimulation site and that transmission from the cortex to this element requires the generation of more than one spike in the cortical neurons. Another possible explanation for this discrepancy may lie in the distinction between sensation and perception. It may be that a stimulus can be sensed and acted upon at one level of the nervous system, but only perceived (come into consciousness) if it elicits activity elsewhere. Old ideas about "subliminal perception" would make sense in this context. (Experiments in "blind sight" form the visual equivalent to this. These experiments are described in Chapter 7.) It is also possible that the somesthetic cortex is not part of the "normal" pathway by which the conscious sensation (perception) is elicited by a stimulus to the skin. The observation that cortical ablations do not eliminate but merely elevate thresholds of sensation begin to make more sense in the light of these considerations.
| Central mechanisms of pain sensations |
Stimulation of the surface of the cortex seldom leads to painful sensations. Neither does removal of any portion of the cerebral cortex eliminate pain sensations; but, this is not to say the cortex plays no role in pain perception. We have already had occasion to point out that removal of the prefrontal lobe, a prefrontal lobotomy or lobectomy, results in a dissociation between the discriminative and affective aspects of pain. When stuck with a pin, such a patient reports that it hurts, but it doesn't bother him. There is also the well-known suppression of pain sensations that occurs as an everyday event, but is more prominent during military battles. A person may be wounded seriously and not be aware of it, or he may suppress being aware of it during concentration on some other activity. Whether this sort of suppression is of cerebral cortical origin is unknown, but it could be.
Pain sensations can be elicited by stimulation in some areas of the thalamus. For example, stimulation in the ventral and posterior parts of VPL and VPM in awake patients evokes excruciatingly painful sensations localized to the appropriate area of the body (appropriate according to the thalamic somatotopic organization). Lesions in this area have sometimes proven effective in eliminating, for a short time, certain forms of spontaneous pain such as causalgias, burning pain caused by damage of a peripheral nerve, and phantom-limb pain, pain experienced in a limb that has been removed (occurs in 5-10% of amputees, especially those with pain in the limb before amputation). Other areas of the thalamus contain cells that respond to noxious stimulation or when stimulated, lead to pain sensations, or, when removed, temporarily reduce or eliminate pain sensation, but as yet we do not have a thorough understanding of this system. In cases of thalamic lesions, pain is alleviated for a short time, but soon returns, often with even greater severity than before the lesion; this condition is termed hyperpathia. To the patient this may mean an endless series of surgical lesions, he may wish had never begun, just to keep ahead of the advancing pain intensity. It has been customary to think of the experience of pain as occurring in the thalamus simply because it is the "highest" center in which it can be recorded, evoked, or blocked.
Gate theory
In most sensory systems there exists a phenomenon known as masking which is an increase in the threshold for perception of a given stimulus caused by the presence of another stimulus. In audition, tones of similar frequencies tend to mask each other. There is only a slight increase in the threshold for detection of a 400 Hz tone in the presence of an 800 Hz tone, but there is considerable increase in threshold for a 750 Hz tone in the presence of an 800 Hz tone. In the olfactory system, strong odors tend to mask weaker odors. Similar masking phenomena occur in the somatosensory system. Touching the skin near a hair will block the sensation evoked by flicking the hair with a pencil point. Also, weak cutaneous stimulation and sometimes even strong stimulation can block or reduce cutaneous pain sensations. (All of these masking phenomena probably do not have the same neural mechanisms, but they are similar phenomenologically.)A number of properties of pain perception (including masking) have led to the proposal of the Gate Theory of Pain.
- counter-irritation, such as lightly rubbing the skin near the painful area, can relieve the pain experienced
- under certain circumstances (for example, in causalgias), gentle stimulation of an injured area can produce intense pain, though it does not produce pain on normal skin
- intense, spontaneous pain often results from pathological conditions, such as tabes dorsalis, in which large myelinated fibers (which don't carry pain signals) are affected preferentially
- pain often results in the ten second period after ischemic pressure-block of a nerve while large fibers fail to conduct
- stimulation of the dorsal columns ipsilateral to the pain often produces relief of pain
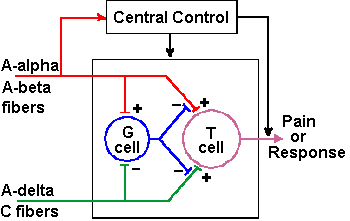 |
Fig. 6-8. The circuit diagram of the gate-control theory of pain perception as proposed by Melzack and Wall (1965) |
To account for these observations, Melzack and Wall proposed that the perception of pain depends not only upon pain signals in small fibers, but also upon the balance of activity in large myelinated mechanoreceptive fibers (A-alpha and A-beta fibers) and small myelinated and unmyelinated nociceptive fibers (A-delta and C fibers). It is important to remember that not all small fibers are nociceptive--some are mechano- or thermoreceptive. Melzack and Wall proposed that, under normal conditions, anything that increases the activity of the large mechanoreceptive fibers tends to reduce pain, and anything that increases the activity of small nociceptive fibers tends to increase pain by changing the balance. Specifically, they proposed the model illustrated in Figure 6-8. According to the theory, the transmission cells [T cells] originally thought to be cells of the spinothalamic tract] activate neural mechanisms that produce pain perception, i.e., T-cell activity is interpreted by the central nervous system as pain. A gate cell, or G cell (originally thought to be cells of the substantia gelatinosa), is capable of modulating the activity in afferent pathways (of both large and small fibers) to the T cell, before it gets to the T cell. The G cell activity is, in turn, modulated by activity in both large and small primary afferent fibers. Specifically, volleys in both large and small fibers excite the T cell (drive its membrane voltage toward the critical firing level), whereas the same volleys in large fibers excite the G cell to discharge and the same volleys in small fibers inhibit the G cell discharge (drive its membrane voltage away from the critical firing level). Activity in the G cell inhibits (reduces or blocks) transmission from both large and small fibers to T cells. Cortical and subcortical influences on the gate control mechanism, called "Central Control" in the figure, are indicated in the diagram of Figure 6-8. This diagram is just one module of many; there are many T and G cells and, of course, a million or so afferent fibers.
How does this theory account for the observations above?
- Lightly rubbing the skin increases the discharges of large myelinated fibers and, in turn, the discharge of the G cell. This results in a decreased transmission through both large and small afferent fibers to the T cell which, of course, is perceived as a reduction in pain sensation because the severity of pain sensation is proportional to the frequency of firing of the T cell
- In causalgias, there can be a selective destruction of the large fibers in the affected nerve. This will result in diminished G cell activity caused by reduced excitation from large fibers and the unopposed inhibition from small fibers, thereby opening the gate and letting the T cell receive excitation through the large and small fiber pathways. The result will be pain evoked by even the lightest touch
- Many C fibers are slightly spontaneously active and, when the gate is open due to large afferent fiber damage, these C fibers are free to excite the T cell. When the excitation builds up enough to bring the membrane potential of the T cell to the critical firing level, spontaneous pain will be experienced
- After an ischemic block of a peripheral nerve, the large fibers recover more slowly than the small fibers. Therefore, there is a period of time when the gate is open due to the absence of the large fiber activity
- The dorsal column fibers are branches of the large myelinated fibers of the medial division of the dorsal root. Stimulation of the dorsal columns sends impulses up (orthodromic) and down (antidromic) the fibers of the columns. When the antidromic impulses reach the point of bifurcation near the dorsal root entry zone, action potentials are set up in both the dorsal root (antidromic) and in the branch that descends into the spinal gray matter (orthodromic). The orthodromic impulses act just like those from the periphery in closing the gate by activating the G cell; thus, the pain is reduced. Small stimulating devices have been implanted in the dorsal columns or sewn to the dura over the dorsal columns of patients with chronic pain. The patient can activate the device himself when the pain recurs. Some success has been reported using this technique.
There was a flurry of excitement when this theory was introduced and with it a great deal of research. The original specifications of the theory assigned to specific cells in the spinal cord the roles of G and T cells. These specific cells do not seem to have all of the properties of G and T cells. In addition, there are some examples of clinical syndromes that do not seem, at first glance, to fit the gate theory. In Friedrich's ataxia--a hereditary disease, symptoms of which are ataxia, speech impairment, lateral curvature of the spine, swaying, irregular movements, and muscle paralysis--there is a preferential loss of large fibers without pain. Similarly, the polyneuropathy associated with renal failure in adults is not associated with complaints of pain, although there is destruction predominantly of large fibers. Nor do some other neuropathies make any sense in any current theory of peripheral nerve involvement in pain sensation. Fabry's disease--a rare phospholipid storage disease-is associated with both pain and the loss of small myelinated fibers, and one hereditary sensory neuropathy is associated with loss of myelinated fibers, preserved C fibers, and analgesia. One should bear in mind that none of these is a disease of peripheral nerves alone. Friedrich's ataxia also involves sclerosis of dorsal and lateral columns of the spinal cord, including the pyramidal tract, and Fabry's disease involves pathological conditions of the cardiovascular and renal systems as well as skin and muscle lesions. The effects on pain of these forms of polyneuropathy are not explained by reference to which peripheral nerve fibers are present or absent. Therefore, it may be the involvement of central pain mechanisms that makes these diseases appear to fit no existing theory.
| According to the Gate Theory, perception of pain depends not only upon pain signals in small fibers, but also upon the balance of activity in large myelinated mechanoreceptive fibers and small myelinated and unmyelinated nociceptive fibers. |
It should be kept in mind that not all pain is of peripheral origin, i.e., some pain does not arise from activity in nociceptors. Causalgias are a class of disorders involving burning pain due to peripheral nerve damage, not receptor damage. The importance of the separation of "central" and "peripheral" pain is indicated by the observation that patients often obtain relief from spontaneous pain of causalgias when the dorsal columns are stimulated, but the same stimulation does not elevate the threshold for pain induced by pinching the skin in the referral area of the causalgias. The stimulus also does not elevate touch and vibration thresholds. It appears that causalgias may have mechanisms different from those of peripheral pain, but how they differ is as yet unknown.
A further indication of this difference between "normal" and "pathological" pain is the result of attempts to close the gate by peripheral stimulation. In patients with causalgias, stimulation of large-diameter fibers has been used to attempt to activate the G cells and close the gate. In 1/3 or more of such patients, partial to complete relief has been obtained, but the same stimulation has no effect on pain due to noxious stimulation of the hand. Pain due to squeezing the Achilles' tendon can be relieved by large fiber stimulation, suggesting that all sorts of "normal" pain may not be the same either. Similarly, morphine can decrease postsurgical tonic pain, but has less effect on acute pain of movement or removal of stitches. Nitrous oxide, on the other hand, depresses both and is perhaps the only true analgesic agent.
Endogenous pain suppression system
It is doubtful that the gate control theory, as it was originally proposed, is adequate to explain all types of pain, but it seems reasonable that some similar gating mechanism(s) are active in pain perception, either in the spinal cord or at some other level of the central nervous system. In fact, there is some evidence for such a mechanism at the brain stem level. Stimulation of the periaqueductal gray matter of the brain stem (Fig. 6-9) produces analgesia in rats sufficient to do abdominal surgery and equivalent to a dose of 50 mg/kg of morphine, but without the muscular rigidity that accompanies such high doses. It blocks withdrawal reflexes due to skin pinch, noxious heat, tooth pulp stimulation (supposedly evoking pure pain sensations), and chemical irritants. The analgesia induced by a few minutes of high frequency stimulation lasts minutes, hours or days but is not a total analgesia because some parts of the body are unaffected. Again, pathological pain is blocked more easily than normal pain.
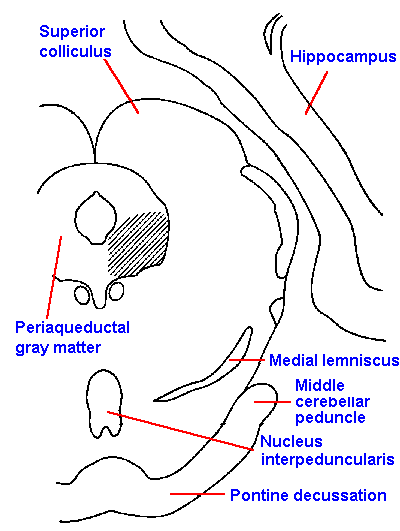 |
Fig. 6-9. The location of stimulus sites within the periaqueductal gray matter (shaded area) at the level of the superior colliculus from which analgesia can be produced in the cat |
Injection of minute amounts of opiate drugs into the region of the periaqueductal gray matter induces strong general analgesia similar to that obtained with systemic morphine. The opiate-antagonist drug, naloxone, blocks the analgesic effects of systemic and microinjected morphine and reduces the effects of periaqueductal gray matter stimulation. The effects of morphine and stimulation are also reduced or blocked by depletion, with parachlorophenylalanine, of serotonin (5 hydroxytryptamine, 5HT), a putative excitatory transmitter substance, and blocked by lesions of the ipsilateral dorsolateral funiculus of the spinal cord. These latter observations prompted a search for a pathway descending to the spinal cord in the dorsolateral funiculus from the region of the periaqueductal gray matter and containing serotonin. Such a pathway originates in the nucleus raphe magnus of the medullary reticular formation. Stimulation within this nucleus also produces analgesia similar to that seen with periaqueductal gray matter stimulation. Subsequently, a synaptic link between the periaqueductal gray matter and the nucleus raphe magnus has been demonstrated, and cells in both nuclei have been shown to be responsive to noxious inputs as well as light tactile stimulation.
At about the same time that stimulation-produced analgesia was being discovered, pharmacological investigations were revealing the presence of receptors in the brain to which opiates bind specifically. This suggested that there were naturally occurring morphine-like compounds in the brain that could bind to these receptors. Subsequently, two pentapeptides, the enkephalins, and certain other peptides, the endorphins, were isolated from brain tissue and shown to have analgesic effects like morphine when injected systemically or into the ventricle. These compounds are found at many places within the central nervous system, but, notably, they occur in the periaqueductal gray matter and the area of the spinal cord from which the spinothalamic tract has been shown to originate. The opiate receptors have also been seen on the terminals of primary afferent neurons in the dorsal horn; some investigators believe these are the terminals of nociceptive axons.
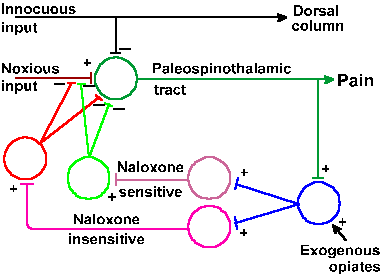 |
Fig. 6-10. The endogenous pain suppression theory includes a negative feedback system in which spinothalamic tract cells (1), activated by noxious input, activate cells of the periaqueductal gray matter (2), which in turn activate descending pathways from the nucleus raphe magnus (3) and the locus ceruleus or nuclei subceruleus and parafacicularis (4). At the segmental spinal cord, interneurons (5 and 6) activated by these descending impulses inhibit further activation of spinothalamic tract cells. Also shown are the sites of action of dorsal column influences and morphine (Basbaum AI and Fields HL: Ann Neurol 4:451-462, 1978). |
From the preceding observations, Basbaum and Fields (1978) proposed what they called an endogenous pain suppression system. The model, shown in Figure 6-10, proposes that noxious stimulation excites nociceptors, which, in turn, excite cells of the spinothalamic tract, giving rise to pain sensations. Spinothalamic fibers excite cells of the periaqueductal gray matter, which, in turn, excite cells of the nucleus raphe magnus. The latter cells can also be excited by nociceptive inputs via other pathways. Axons of raphe magnus neurons descend in the dorsolateral funiculus, and their activity excites interneurons in the spinal cord that contain endorphins as a transmitter substance. When released, the endorphins block the release of transmitter substance (possibly substance P, another peptide compound) onto spinothalamic tract cells, preventing or reducing further nociceptive discharges in them. This is an example of a negative feedback loop in which the output of a group of cells, in this case the spinothalamic tract cells, is used to reduce its input, in this case the amount of nociceptive input. The effect of the system is to reduce subsequent pain sensation. The model suggests that morphine acts by exciting the system at the periaqueductal gray matter.
| The endogenous pain suppression system proposes that activity that leads to painful sensations also leads to the prevention or reduction of further pain sensations. |
This model explains the effects of morphine (systemically and locally injected), of periaqueductal gray matter and nucleus raphe magnus stimulation, and of endorphins on pain sensitivity, at least in part. However, naloxone administration and serotonin depletion only partially block the effect of periaqueductal gray matter stimulation, implying that there must be an additional mechanism for this effect.
This model requires that periaqueductal gray matter stimulation and morphine administration actually result in inhibition of nociceptive activity in spinal cord neurons. Studies have indicated that, in the rat, injection of morphine into the periaqueductal gray matter leads to decreased responsiveness to noxious stimulation in some lumbar spinal cord neurons; others increase their activity or are not influenced. In some of the inhibited cells, naloxone returned the cell discharge to normal despite continued noxious stimulation. Periaqueductal gray matter stimulation does not influence the discharge of most cells that respond to mechanical, non-noxious stimulation. The situations in the monkey and cat are similar, but not quite as neat. Stimulation of the raphe magnus nucleus inhibits both nociceptive and non-nociceptive, tactile activity in spinothalamic neurons. Thus, it is clear that not all nociceptive activity is inhibited by stimulation in regions that produce analgesia; however, not all nociceptive activity is necessarily associated with pain perception. As we shall see when we discuss synaptic transmission (Chapter 13), it is not necessary to reduce nociceptive inputs to a cell to zero in order to bring the membrane potential below critical firing level, preventing its discharge. Still, the link between the cellular events in the spinal cord and the analgesia observed has yet to be established, so the model is tentative, but it has already led to new pain treatments.
Referred pain
It is customary to classify visceral sensations as either organic sensations or as pain sensations. Organic sensations are those that signal a bodily need and that usually lead to an activity designed to satisfy that need. Hunger sensations are an example. All fibers that carry visceral sensation travel the viscera in the company of autonomic efferent fibers and enter the spinal cord through the deep nerve trunks and the ventral ramus of the dorsal root.
Visceral pain is peculiar in that it is often felt to be coming from the surface of the body, not from the organ that contains the pain receptors. We say that the pain is referred to the site on the skin. The area of the referral is always in the dermatome innervated by the same dorsal root(s) as those supplying the irritated structure in the viscera. This is a case where knowledge of the dermatomes becomes an important diagnostic tool. Angina pectoris is a classic example in which pain caused by anoxia of the myocardium is referred to the chest and arm. The pain fibers from the myocardium originate in the dorsal root ganglia T1-T5, which have dermatomes on the chest from below the nipple up to about the level of the clavicle and also down the posterior aspect of the arm. It serves a clinician well to memorize this distribution, because chances are he will see it again.
There are a number of other examples of referred pain. Regurgitation of acid into the esophagus is sensed as burning pain localized to the region of the lower end of the sternum ("heart burn"), whereas referred pain for inflammation of the appendix is experienced in the lower right abdominal quadrant. It should be noted that not all visceral pain is referred. Appendicitis is also accompanied by diffuse pain near the midline at the epigastric level, and a myocardial infarction is usually accompanied by deeper aching in the chest in the vicinity of the heart.
| Referred pain probably occurs because visceral pain fibers converge onto the same pain pathways as do non-visceral or cutaneous pain fibers. |
Referral of pain is not limited to visceral organs. Pain in some nonvisceral organs such as the peritoneum and the diaphragm is also referred to the appropriate dermatomes. The pain fibers from the central region of the diaphragm travel in the phrenic nerve to enter the spinal cord at C3 and C4. Thus, pain in the diaphragm, for example, caused by subphrenic abscess, is referred to the region of the neck and shoulders. Referral of pain from the diaphragm also occurs following a gynecological test to determine the patency of the fallopian tubes, Rubin's test. In this test, CO2 is forced into the uterus and out through the tubes, and if they are patent, the bubble of gas escapes past the fimbria into the peritoneal cavity. It causes no sensation in the horizontal position, but upon rising the patients often report pain in the shoulder caused by the localization of the bubble beneath the diaphragm. The pain subsides, of course, as the gas is absorbed.
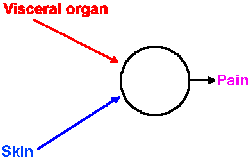 |
Fig. 6-11. Schematic diagram showing convergence of input from pain fibers originating in skin and visceral organs onto cells that signal pain to the central nervous system. |
The mechanism of the referral is probably the same for visceral and other somatic pain. The visceral pain afferent fibers likely terminate on the same central cells and influence them in the same way as the cutaneous and muscle pain fibers of that dermatome (Fig. 6-11). Through experience, the discharge of those central cells has become associated with pain of the body surface, visceral pain being an infrequent event. The brain thus interprets activity in those cells as pain at the body surface, not deep pain. Whatever the nature and the location of the central pain mechanism, it "knows" only that the central cell has discharged; it does not know what made it discharge.
Pain treatment
The most common treatment for pain is the administration of so-called analgesic drugs. This is, perhaps, an unfortunate term, because few of these drugs produce analgesia in the sense of insensitivity to pain. In most cases, they produce pain relief--aspirin relieves some headaches--but pain sensitivity is not lowered. The patient still responds with the same sensitivity to noxious heat, damaging mechanical or chemical stimulation or electrical stimulation. As already mentioned, even morphine does not produce analgesia. General anesthetics may produce analgesia, or they may not. We only know that the patient does not report experiencing pain under the influence of a general anesthetic agent, but withdrawal reflexes and reflex changes in blood pressure still occur in response to painful stimulation. Perhaps the patient felt pain, but just didn't remember. No one can say with certainty. We will see that most surgical treatments for pain also produce pain relief, not analgesia, but, of course, that is what the patient really wants.
Chronic pain has been treated with a number of destructive surgical interventions. Dorsal rhizotomies have been performed for chronic pain owing to malignancies, vertebral disk disease, spinal trauma and other causes, but the success rate (complete pain relief) is less than 30%. There are, of course, fibers other than nociceptive fibers in the dorsal roots, and losses occur in other than pain sensations. The use of this procedure is further complicated by the fact that, even though a nerve block by local anesthetics may alleviate pain totally, subsequent rhizotomy of the same roots may be without effect. A similar treatment for trigeminal neuralgia or tic douloureux (a severe, episodic pain in the face associated with large-fiber damage and triggered by gentle but not intense stimulation of the face), involving thermocoagulation of the trigeminal roots or ganglia within the foramen ovale, has achieved a success rate of 90% to 100%, though up to 17% required more than one treatment. This treatment has the advantage of preserving touch sensation in the analgesic field in many cases, but pain still recurs in 22% of cases. The cause of the relief may not be destruction of nociceptive fibers in every case, because a mistaken coagulation of the wrong trigeminal (the one contralateral to the pain) led to pain relief for two years in one patient.
The notion that the spinothalamic tract was the pain pathway provoked a great many anterolateral cordotomies (or spinothalamic tractotomies) for the relief of chronic pain. Initially, these were done surgically, but later they were done percutaneously by inserting metal electrodes into the spinal cord (without a laminectomy) and coagulating a portion of the anterolateral quadrant. As mentioned before, this procedure never produces permanent pain relief. A dysesthesia, a persistent, painful sensation produced by gentle stimulation, usually develops sometime after a cordotomy, and it worsens with time. For these reasons, cordotomies are seldom done except in terminal-disease cases.
It is possible to make small lesions in specific structures within the brain by passing a small amount of current through an electrode placed in that structure. The current produces heat that destroys the nearby cells. Such electrodes can be placed accurately using stereotaxic coordinates, a Cartesian coordinate system, read from an atlas and referenced to particular points on the head. The position of the electrode can be checked using known properties of the cells in the region, e.g., the cells of the nucleus ventralis posterolateralis of the thalamus are known to discharge in response to skin stimulation, and by X-ray imaging. Lesions have been made in the various relays for nociceptive information in the thalamus or mesencephalon. But, everyone who has used this type of intervention has found the same result: there is initial relief followed by rapid return of pain. Even large and bilateral lesions fail to relieve pain, and usually lead to exacerbation of preoperative pain or a dysesthesia, that is worse than the initial complaint. At present, there is little justification for thalamotomy in pain treatment.
The causalgias are burning pains associated with peripheral nerve damage, often associated with penetrating wounds, and they are frequently exacerbated or triggered by emotional and thermal stimulation. These pains can also be triggered by light touch or any stimulus that elicits a startle reflex. The involvement of emotion suggests that the sympathetic nervous system may play a role in causalgia; in cases where pain is exacerbated by such stimuli, sympathectomy has produced at least some pain relief. Paravertebral sympathetic nerve blocks are good indicators of the success of sympathectomy. This form of treatment has also been used for pain relief in disease confined to the visceral capsule, e.g., angina pectoris, aortic aneurysms, and postcholecystectomy neuralgia, but seldom is effective for pain due to malignancy. For causalgia with emotional exacerbation, good, long-term pain relief has been obtained in up to 83% of cases where sympathectomies have been performed. The mechanism of the pain relief is not known, but (1) abolition of vasomotor and sudomotor efferent discharge, with relief from ischemia, and (2) interruption of accessory sensory pathways carrying pain discharges from blood vessels have been suggested as explanations.
Pain due to hormone-dependent tumors is usually not responsive to surgical or nerve-block treatments, because the tumors are commonly metastatic and distributed over wide areas or in different organs. Some patients suffering from this sort of chronic pain have been treated by chemical hypophysectomy. A small amount of ethanol injected in the region of the pituitary produced complete, immediate and long-lasting relief in 88% of the patients in one study, and some of the remaining patients also received relief when the procedure was repeated. The treatment not only results in pain relief, but there is usually an arrest or regression of the metastasis. Two hypotheses suggest that (1) hormones potentiate transmission through sensory pathways, and removal of hormones reduces pain discharge transmission, and (2) ethanol works not only on the pituitary, but also diffuses to surrounding tissues that are in the pain pathway. Hormones are known to potentiate sensory transmission, but potentiation of nociceptive sensory transmission has not been studied.
Percutaneous stimulation and dorsal column stimulation to activate large-diameter fibers have already been mentioned for pain treatment. Stimulation of a peripheral nerve, central to nerve damage in a patient with causalgia, produces immediate pain relief that outlasts the duration of stimulation. Two minutes of stimulation at 100 Hz has produced relief lasting up to seven hours; eventually pain returned, sometimes with an overshoot (it was worse than before stimulation), but repeated stimulation produced repeated relief. Such stimulation has been effective in 70-85% of the patients in different clinics. Percutaneous stimulation has been used to treat headache and pain due to burns and abrasions; relief is more frequent for this type of new pain than for chronic pain. In a group of patients with chronic low back pain, 39% obtained complete relief, 28% obtained some relief, but 33% obtained no relief. There are three hypotheses advanced to explain pain relief by percutaneous stimulation. Closure of the pain gate in the gate control theory is one explanation. Blockage of transmission in small fibers as a result of repeated, high-frequency stimulation is another. Masking of pain sensation by evoked paresthesias is yet another explanation. This last alternative does not seem to explain all cases, because pain usually fades out gradually during stimulation and gradually reappears, whereas the paresthesias appear abruptly with stimulation and disappear before pain recurs.
Dorsal column stimulation has been used most often in treatment of spinal column disk pain. Some patients experience paresthesias and pain relief during stimulation, but both disappear when stimulation ends. In others, paresthesias may last up to 30 minutes longer than stimulation, and pain relief up to four hours longer. Pin-prick pain, light touch, and vibration sensation, and stereognosis are unaffected by dorsal column stimulation, as they are by dorsal column lesions (Friedman, Nashold, Somjen, 1974). Dorsal column stimulation cannot relieve postoperative pain caused by implantation of the stimulator or pain due to long-bone fracture, but it has been effective in 81% of the cases of disk pain.
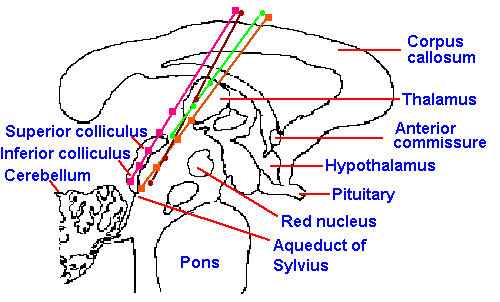 |
Fig. 6-12. Stimulation sites in human patients from which pain relief has been obtained. Electrode tracks are shown, along which the stimulation sites are marked with different symbols for each patient. These tracks traverse the periventricular gray area along the medial aspect of the nucleus parafascicularis of the thalamus. (Richardson DE, Akil H: J Neurosurg 47:178-183, 1977) |
The experimental observations of the effects of stimulation of the periaqueductal gray matter in rats have now been extended to cats and monkeys and, finally, to man. In pain patients, various forms of chronic pain, including disk pain, tumor pain and phantom-limb pain, have been relieved by such stimulation, without any apparent influence on other sensory modalities (vision, etc.) or submodalities (light touch) or changes in motor or intellectual activity. In some cases, acute experimental pain has also been blocked, but usually with more difficulty and for shorter times. The diencephalic periventricular gray matter, the pretectal nucleus, and some hypothalamic sites have also produced analgesia when stimulated (Fig. 6-12). Medial periventricular thalamic structures are preferred targets for stimulation in humans, because periaqueductal stimulation usually produces unpleasant sensations, including nystagmus and nausea. Chronic pains beginning after implantation of stimulating electrodes may not be influenced by stimulation; electrically induced, radiant heat-induced, and ischemic exercise-induced pains may also be unaffected. When patients are allowed to control stimulation, a tolerance, which generalizes to opiate drugs, may develop in 4-5 weeks, but if use is limited, this tolerance does not develop. Bilateral pain relief occurs with unilateral stimulation. About 75% of the patients treated with this kind of stimulation (in one study) received at least some relief from pain; many received complete relief. In 5 of 6 patients, naloxone administration reversed the relief that resulted from stimulation (Richardson and Akil, 1977). In another study, similar stimulation had no effect on pain sensation in any patients (Mazars, Merienne, and Cioloca, 1979). This form of treatment is not much used because of the advent of intraspinal and intraventricular administration of opioids. It is, however, still used in treatment of deafferentation pain where it successfully yields long-term relief in about 30% of patients (Gybels, Kupers and Nuttin, 1993).
The treatment of choice for pain is not always clear; nor is it clear that a single treatment is good for all types of pain. Early attempts at treating pain with a new procedure are often more effective than later attempts. One should guard against being overly optimistic about the effectiveness of new treatments. In a review, Shealy (1974) concluded that "traditional destructive procedures-cordotomy, cingulumotomy, rhizotomy-are not only almost useless (except in cancer) but they cause far more harm than good, and they interfere with success of other procedures." In cancer, the patient usually does not survive long enough to suffer the harm of these procedures. The mechanism of action is not known for any pain treatment. Most do not produce true analgesia but only pain relief. Many produce only transient relief and, for none, has an appropriate control procedure been done to allow evaluation of the effectiveness of the treatment itself. For example, trigeminal coagulation produces pain relief in most cases of trigeminal neuralgia, but simple manipulations without destruction can also produce relief. Pain is, indeed, a curious phenomenon!
Acupuncture
The practice of acupuncture in treatment of disorders is very old in China, but interest in it has been stirred in this country by demonstration that it can be used for analgesia. The technique essentially is to insert metal needles through the skin at certain critical points and then to either manipulate them or stimulate through them using mild electrical currents. Exact sites and number of sites are determined by the position in which the analgesia is desired. The patient usually reports a tingling, pricking or numbing sensation during stimulation of the needles with a stimulus strength that is great enough to excite A fibers and perhaps the largest A fibers. Under this intervention, everything from minor dental work to major abdominal and thoracic surgery is performed without anesthetic agents or their side-effects. Two advantages of the procedure are a more rapid recovery and the absence of any need for close supervision during recovery.One is at first tempted to interpret the acupuncture phenomenon in terms of the gate theory. This temptation is brought on by the rather selective excitation of large myelinated fibers by the needles and their stimulation. However, in the gate theory, the large and small myelinated fibers operating the gate supposedly enter the same spinal cord segment. Conversely, the acupuncture needles are often placed in the hand or ear lobe for procedures in the lower abdomen, a separation of many segments for entry of the appropriate afferent fibers. Indeed, some variation of the gate theory could be invoked to explain the phenomenon, but pathways which might be involved are not known at this time.
In China, acupuncture is used in about 30% of the hospital surgical procedures. If the procedure is so useful, why isn't it used in 100%? The most likely answer is that it only works in 30% of the cases. This is about the same proportion of cases that receive relief from placebo treatments or have surgical procedures under hypnosis. It is reasonable to suggest that the monotonous stimulation has a somewhat hypnotic effect upon the patient. On the other hand, acupuncture has been reported to be effective on animals and small children, suggesting that hypnosis is not the only explanation, if it is an explanation at all.
We should recall that some superficial pains can be blocked by intense stimulation or counter-irritation. This intense stimulation can be in the form of dry needling, intense cold or injection of normal saline. Treatment with mustard plasters and blistering agents may also be considered the same form of treatment, sometimes called hyperstimulation analgesia. It seems paradoxical that intense stimulation should relieve pain from intense stimulation, but, in some cases, it works. Some investigators have suggested that acupuncture may be just another case of hyperstimulation analgesia. The acupuncture needles apparently have no effect on pain unless they are moved or an electrical current is applied, implying a significant input to the CNS is required for the analgesia. However, analgesia is usually produced by hyperstimulation near the area of injury; acupuncture needles are effective at a distance.
| Summary |
Afferent fibers enter the spinal cord through the dorsal roots and enter the major ascending pathways by synapsing at segmental levels or by ascending directly in the dorsal columns or both. Some second-order neurons at the segmental level cross to the contralateral ventral white matter and ascend as the spinothalamic tract. Fibers from the face enter through the trigeminal nerve and synapse in the trigeminal nuclei. Second-order neurons in the dorsal column system arise from the dorsal column nuclei. Third-order neurons in all three pathways arise from the thalamus and ascend to the cerebral cortex. Sensory submodalities usually associated with the dorsal column system are touch, pressure, vibration, and position sense; those usually associated with the spinothalamic system are pain, crude touch, deep pressure, and temperature. Fibers in both the dorsal columns and the spinothalamic tract can respond to light tactile stimulation, noxious stimulation, or both light tactile and noxious stimulation. Sensory fibers do not distribute randomly in any part of the nervous system, but order themselves in such a way as to preserve spatial information in their position; this is somatotopic organization. Dermatomes are the composite receptive fields of all primary afferent fibers entering a given dorsal root and knowledge of their distributions is important in physical diagnosis. Cortical lesions do not abolish somatic sensation, but elevate thresholds and interfere with use of somatic information in solving problems. The gate control theory of pain suggests that whether pain is experienced in a given situation depends upon the balance of activity between large myelinated mechanoreceptive fibers, and small myelinated and unmyelinated nociceptive fibers. An endogenous pain suppression system has been suggested to involve the periaqueductal gray matter, the raphe magnus nucleus and descending pathways to the spinal cord. Pain relief does follow stimulation at these sites, but the mechanism is still being studied. Pain has been treated successfully with drugs, surgery, and stimulation techniques.
| Suggested Reading |
- Angaut-Petit D: The dorsal column system. II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res 22: 471-493, 1975.
- Basbaum AI, Fields HI: Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 4: 451-462, 1978.
- Clark FJ: Central projections of sensory fibers from the cat knee joint. J Neurobiol 3: 101-110, 1972.
- Corkin S, Milner B, Rasmussen T: Somatosensory thresholds. Contrasting effects of postcentral-gyrus and posterior parietal-lobe excisions. Arch Neurol 23: 41-58, 1970.
- Dennis SG, Melzack R: Pain-signalling systems in the dorsal and ventral spinal cord. Pain 4:97-132, 1977.
- Friedman H, Nashold BS Jr, Somjen G: Physiological effects of dorsal column stimulation. In Bonica JJ (ed): Advances in Neurology, Vol. 4, Pain. pp. 769-773, New York, Raven Press, 1974.
- Gybels J, Kupers R, Nuttin B: Therapeutic stereotactic procedures on the thlamus for pain. Acta Neurochir (Wien) 124:19-22, 1993.
- Iggo A (ed): Handbook of Sensory Physiology, Vol II, Somatosensory System. Berlin, Springer, 1973.
- Kaas JH: Topographic maps are fundamental to sensory processing. Brain Res. Bull. 44:107-112, 1997.
- King RB: Principles of pain management. A short review. J Neurosurg 50:554-559, 1979.
- Mazars G, Merienne L, Cioloca C: Effets des soi-disant stimulations de la substance grise peri-acqueductale. Neurochirurgie 25: 96-100, 1979.
- Melzack R, Wall PD: Pain mechanism: a new theory. Science l50: 971-979, 1965.
- Nathan PW: The gate-control theory of pain. A critical review. Brain 99: 123-158, 1976.
- Nathan PW, Rudge P: Testing the gate-control theory of pain in man. J Neurol Neurosurg Psychiat 37: 1366-1372, 1974.
- Richardson DE, Akil H: Pain reduction by electrical brain stimulation in man. I. Acute administration in periaqueductal and periventricular sites. J Neurosurg 47: 178-183, 1977.
- Shealy CN: Six years' experience with electrical stimulation for control of pain, In Bonica JJ (ed): Advances in Neurology, Vol. 4, Pain. pp. 775-782, New York, Raven Press, 1974.
- Trevino DL, Coulter JD, Maunz RA, Willis WD: Location and functional properties of spinothalamic cells in the monkey, In Bonica JJ (ed): Advances in Neurology, Vol. 4, Pain. pp. 167-170, New York, Raven Press, 1974.
- Weinberg RJ: Are topographic maps fundamental to sensory processing? Brain Res. Bull. 44: 113-116, 1997.
- Woolsey CN: Cortical localization as defined by evoked potential and electrical stimulation studies, In Schaltenbrand G, Woolsey CN (eds): Cerebral Localization and Organization, pp. 17-26, Madison, WI, Univ of Wisconsin Press, 1964.
Footnote:
1. Dorsal column fibers are spatially distant from spinothalamic tract fibers, but fibers in other tracts may well be cut with a spinothalamic tractotomy.
[TOC] [Chapter 7] [Glossary] [Index] [Abbreviations]