 Chapter 14
Chapter 14 
MUSCLE CONTRACTION
It is impossible to overemphasize the importance of muscle in vertebrates. The very life style of every one demands movement, impossible without muscle. In fact, in man about 40% of the body mass is striated muscle, making it the most abundant tissue. Striated muscle is so named because of its characteristic cross-striped appearance. Most striated muscle is skeletal muscle, involved in rotation of bones around joints and therefore responsible for most of the movements of which we are aware. Other striated muscles move the eyes and serve as valves to check the flow of blood or other fluids, e.g., the bulbospongiosus aids erection of the penis or clitoris by compressing the deep dorsal vein. Cardiac muscle is also striated in appearance, but it differs significantly from other striated muscle in both its structure and its behavior. Still other muscles, called smooth muscles, lack the characteristic cross-striations, but contain the same contractile proteins. The smooth muscles are important as linings of the gastrointestinal tract that churn and propel food through the tract, as linings of blood vessels that control their diameters and thus flow through them, as valves that control the passage of gases and fluids in the body, and as controllers at many other places in the body.
Of the three types of muscle, skeletal and cardiac muscle have been studied most thoroughly. It is presumed that the mechanism of contraction is the same for both types and only the details of initiating and controlling the contraction differ. Not all striated muscle, however, behaves in the same way. For example, skeletal muscles of vertebrates all appear to initiate contractions with sodium spikes, whereas striated muscles of some invertebrates initiate contractions with calcium spikes. We will confine our discussion primarily to vertebrate skeletal muscle, pointing out the distinctive features of structure and function of cardiac and smooth muscle.
| Muscle structure |
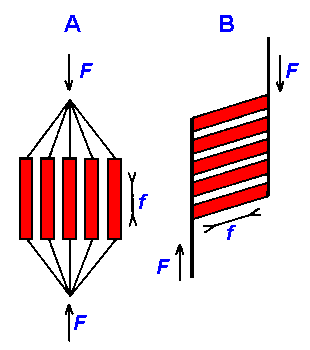 |
Fig. 14-1. Two arrangements of muscle fibers within a muscle. A. Parallel arrangement: Tendons are lines radiating from rectangles (muscle fibers) at each end. B. Pennate arrangement: Tendons are vertical lines extending from the two sides of the parallelogram. Double headed arrows (f) indicate direction of force exerted by individual muscle fibers; single-headed arrows (F) indicate direction of force exerted by whole muscle. (Zierler KL: Mechansims of muscle contraction and its energetics. In: Mountcastle VB [ed]: Medical Physiology. 13th ed, Vol. 1. St. Louis, C.V. Mosby, 1974). |
Skeletal muscles are composed of masses of fibers, each an individual cell. There are several types of muscles, each with different arrangements of fibers, but these can be divided into two major classes: those with fibers arranged in parallel and those with a pennate arrangement. Figure 14-1 shows these two classes. In the parallel arrangement (A), each muscle fiber, or a small group of fibers, is attached to its own tendon, the tendons converging on a common point(1). The muscle fibers are side-by-side, i.e., in parallel, but the name of the class comes from the fact that the muscle fibers shorten in a direction (double headed arrow, f) parallel to the direction of shortening of the muscle (single-headed arrow, F).
The pennate muscle fibers (B) attach to a common tendon, so that the direction of shortening of the individual fibers (double-headed arrow, f) is different from the direction of shortening of the whole muscle (single-headed arrow, F). As a result, the pennate muscle cannot shorten as much as the parallel muscle. Pennate muscles are located in positions requiring small but powerful movements; parallel muscles are located in positions requiring longer movements with less power or faster movements.
Muscles, fibrils and filaments
To understand how a muscle works it is necessary to understand the fine-structure of muscle cells because it is the internal parts of the cells that do the work. The relevant internal structures are the myofibrils, the myofilaments and the sarcoplasmic reticulum. Muscles are composed of muscle fibers; fibers are composed (in part) of myofibrils; and myofibrils are composed of myofilaments. Skeletal muscles have a characteristic striated appearance because the myofibrils are characteristically striated and because the myofibrils are more or less in register (the same stripes are lined up). The myofibrils are striated because the myofilaments are not homogeneously distributed within them, but rather occur in regular, repeating arrays.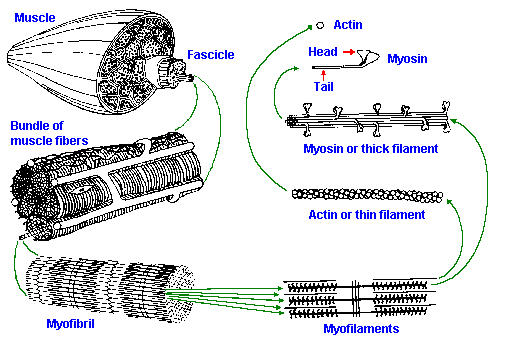
|
Fig. 14-2. Levels of organization within a skeletal muscle, including (counterclockwise from top left) whole muscle and fascicles, bundles of muscle fibers, myofibrils, thin and thick filaments, and myosin and actin molecules. (Warwick R, Williams PL [ed]: Grays' Anatomy. 35th British ed, Edinburgh, Churchill Livingston, 1973; modified from a drawing by Professor D Fawcett) |
Myofibrils
Figure 14-2 shows, on the left, the whole muscle, a bundle of muscle fibers, and their subunits, the myofibrils. Note the striated appearance of all three. Each muscle fiber contains about 1000 myofibrils that are 1 m in diameter and run the length of the fiber. Myofibrils have no membrane, being simply surrounded with cytoplasm.The cross-striations of the myofibrils are serially repeating units called sarcomeres. A sarcomere can be from 1.5-3.5 m in length, depending upon the contractile state of the muscle, and it is bounded on each end by a disc, called the Z disc or Z line. Each sarcomere contains an anisotropic (doubly refractive, therefore dark in phase microscopy) band bounded by two isotropic (singly refractive, therefore light) bands. The anisotropic band is called the A band; the isotropic band is called the I band. Actually, each sarcomere contains two half-I bands (one at each end) because a single I band straddles the Z line and therefore is part of two adjacent sarcomeres. In the center of the A band, there is a lighter region known as the H zone or H band. During contraction the A band does not change length(2), though the sarcomere shortens, the distance between Z lines lessens, and the I and H bands narrow. Any theory of muscle contraction must account for these observations.
The myofibrils, as shown in Figure 14-2, are composed of proteinaceous structures called myofilaments. One filament is thick, about 11 nm in diameter and 1.5 m long, whereas the other is thin, 5 nm in diameter and 1 m long. These filaments are referred to as the thick filaments and thin filaments, respectively. Thick filaments are made up of several hundred myosin molecules, proteins of a molecular weight of about 500,000, and some other minor proteins whose function is unknown. The myosin molecule has a tail region that is rodlike, and head region, with two globular subunits projecting out at approximately right angles with the filament. The structure has been likened to two golf clubs with their shafts twisted together. Drawings of a myosin molecule, and its position within the thick filaments are shown in Figure 14-2. The myosin molecules of thick filaments are arranged in a sheaf with heads oriented toward each end and tails toward the center. Each subsequent myosin molecule attaches 14 nm further toward the end of the filament, and its head is rotated 60 around the filament from its predecessor. Thus, the thick filament is studded with projections except at its center, which contains only myosin tails. Note that myosin molecules at opposite ends of the thick filament are oriented in opposite directions–sort of like a bundle of unsorted golf clubs, some with heads at the right end, some with heads on the left. The thick filaments are coincident with the A band of the sarcomere.
| Troponin and tropomyosin are regulatory proteins that allow the muscle to shorten in the presence of Ca++. |
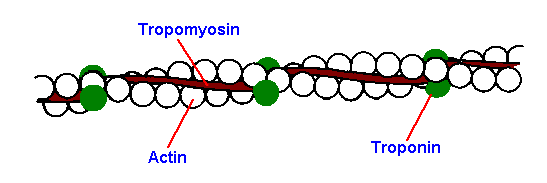
Each thin filament contains three protein molecules: actin, troponin, and tropomyosin. A single thin filament is composed of 300 to 400 actin molecules and 40 to 60 troponin and tropomyosin molecules. Actin is a small, nearly spherical molecule that is arranged in the filament into two helical strands, as shown in Figure 14-2, with about 13 actin molecules per complete turn of the helix. Troponin and tropomyosin are sometimes called regulator proteins because of their central role in regulating muscle contraction. Tropomyosin is a filamentous protein that is thought to form two strands that lie in the grooves formed between the actin strands. Troponin, a globular protein, binds to tropomyosin at only one site and therefore is thought to sit astride the tropomyosin molecule strand at regular intervals approximating 40 nm. Figure 14-4 shows the relationships between the three proteins as they are currently thought to exist. The thin filaments attach to the Z disc, a flat protein structure. Thin filaments may be connected end-to-end in the H band by slender threadlike processes.
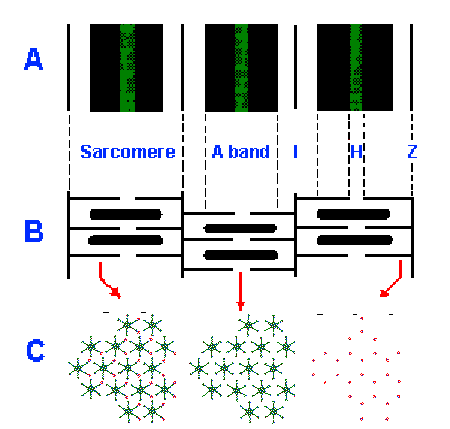
|
Fig. 14-3. Organization of the sarcomeres. A. Pattern of cross-striation in skeletal muscle with bands labeled. B. Arrangement of thick and thin filaments that accounts for the pattern of cross-striations. C. Hexagonal arrays of thick and thin filaments in cross sections through the sarcomere in the A band, H band and I band. |
The relatively high anisotropicity of the A
band results from the presence of both thin and
thick filaments (shown in longitudinal section in
the upper part and cross-section in the lower part
of Fig. 14-3). The I band is only slightly anisotropic because it contains only thin filaments. The
H band is not optically as dense as the rest of the A band because it does not contain any thin
filaments when the muscle is at rest. As can be seen in the cross section of Figure 14-3, the thin
filaments are organized into regular hexagonal arrays within the myofibrils, with a thick filament
at the center of each array in the A band. Three thick filaments are equidistant from each thin
filament, whereas six thin filaments are equidistant from each thick filament as shown in the left
panel. A cross section through the I band shows only the thin filament array; a section through
the H band shows only the thick filament array (plus the slender, thread-like processes
interconnection thin filaments).
|
Fig. 14-4. Thin filament structure. Proposed structure of thin filament with relative positions of actin, troponin and tropomyosin indicated. (Ebashi S, Endo M, Ohtsuki I: Quart Rev Biophys 2:351-384, 1969) |
The myosin heads project out from the thick filaments toward the thin filaments at intervals of about 43 nm measured in a line parallel to an adjacent thin filament. Because they are staggered around the thin filament at 60 intervals, each projects in the direction of a thin filament, and each thin filament has projections toward it from three thick filaments. These projections have been termed either cross-bridges or cross-projections, depending upon whether the heads are thought to contact and bind thin filaments or not. As we shall see, there are two schools of thought, in fact, two different mechanisms proposed to account for the generation of the mechanical force of contraction.
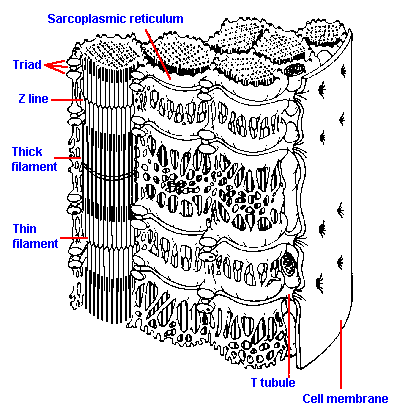
|
Fig. 14-5. Three-dimensional reconstruction of skeletal muscle illustrating organizatin of myofibrils, sarcoplasmic reticulum and T tubules. (Warwick R, Williams PL [ed]: Gray's Anatomy, 35th British ed, Philadelphia, W.B. Saunders, 1973) |
T tubules and sarcoplasmic reticulum
Muscle cells have a unique membrane structure, called the transverse tubule or simply the T tubule. The T tubule is an invagination of the muscle membrane, much like the invagination produced in a balloon by pushing a finger into its side without puncturing it, but the T tubules are long and tortuous. The T tubule system forms a ring around every myofibril either at the Z line, in which case there is one per sarcomere, or at the A-I-band junction, in which case there are two per sarcomere. These perifibrillar rings are inter-connected, forming a kind of honeycomb arrangement, as shown in Figure 14-5. The position of the T tubule with respect to the sarcomere is somewhat species specific; frog skeletal muscle has only one tubule per sarcomere, whereas human skeletal muscle has two. It should be noted that in human cardiac muscle there is only one tubule per sarcomere as shown in Figure 14-17. The inside of the T tubule is continuous with the extracellular space and presumably contains a fluid like extracellular fluid, but, because the tubular space is small and not well stirred, it is likely that ionic movements across the tubule membrane produce significant changes in ionic concentration, at least on a short-term basis.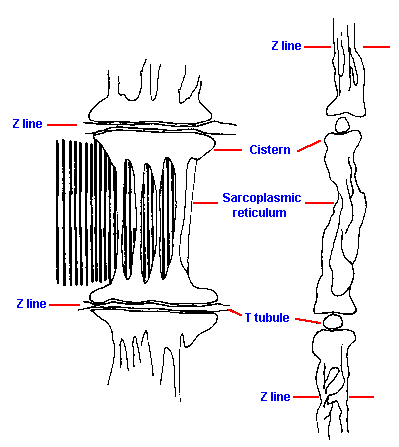
|
Fig. 14-6. Fortuitous sections through triads, one at right angles to the other. Relative positions of T tubules and sarcoplasmic reticula at the triad are shown clearly. |
Another intracellular structure with special significance for contraction is the sarcoplasmic reticulum, the muscle cell version of the endoplasmic reticulum. The sarcoplasmic reticulum is made up of tubules that run parallel to the sarcomeres from T tubule to T tubule (see Figures 14-5 and 14-17); thus, there are two sets of sarcoplasmic reticulum tubules per sarcomere in muscles with two sets of T tubules per sarcomere. The sarcoplasmic reticulum is a sack with its ends expanded (the cisternae) adjacent to the T tubules and with narrow, longitudinal channels connecting these expansions, one at each end. In fortuitous sections, one can find a section of T tubule bounded on two sides by sort of dumb-bell-shaped sarcoplasmic reticula as illustrated in Figure 14-6. The T tubule with its two adjacent regions of sarcoplasmic reticulum is often called a triad. Because the T tubules and the cisternae of the sarcoplasmic reticulum run together for such a long way, a large proportion of the sarcoplasmic reticulum is in contact with the sarcolemma. There is a space of about 12 nm between the membranes which, in electron micrographs, appears to be traversed at regular intervals by structures that have been suggested to be channels coupling the T tubule with the sarcoplasmic reticulum. However, large molecules such as ferritin cannot cross between the two structures, and the lumen of the sarcoplasmic reticulum contains a fluid like sarcoplasm, not like extracellular fluid. In addition, electrical measurements indicate that the sarcoplasmic reticulum does not communicate with the T tubule through low resistance pathways.
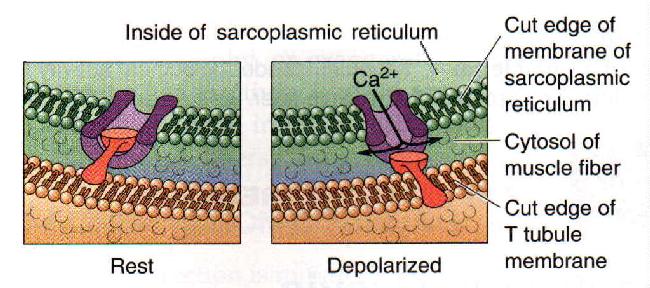
|
Fig. 14-7. A model of the opening of the Ca channel by the action potential is the T tubule of the skeletal muscle fiber. |
One model has a mechanical plug that closes the Ca channel, preventing calcium from leaving the sarcoplasmic reticulum. Hypopolarization of the T tubule somehow pulls the plug out of the channel opening, allowing Ca to enter the sarcoplasm. In Figure 14-7, the plug is show in red (ladle-shaped piece) and the Ca channel in purple (cut cylinder). Presumably, the plug is a dipole whose position is altered by altering the membrane polarization. In any case, Ca efflux from the sarcoplasmic reticulum starts the muscle contraction.
The sarcoplasmic reticulum serves as a repository for Ca++. In rested muscle, Ca++ is found in high concentration in the cisternae at the triad. In recently active muscle, the calcium is found in the narrowed, longitudinal portion from which it moves to the triad as time passes. During contraction, Ca++ is found in high concentration outside the sarcoplasmic reticulum among the myofilaments.
| Sliding filament model |
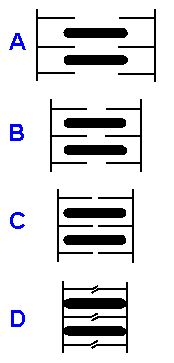
|
Fig. 14-8. Sliding filament model of muscle contraction. A single sarcomere is shown stretched, with little overlap between thick and thin filaments (A), with greater overlap (B), with complete overlap (C), and extremely shortened with thin filaments shown buckled (D). Another possibility, not shown, is that at extreme shortening, the buckling occurs at the Z line. |
Observations that during muscle contraction, the sarcomere, the I and H bands become narrower, while the A band does not, coupled with the observation that thick and thin filaments do not shorten (although at very short lengths the thin filaments may either push through the Z line or fold up like an accordion), suggested the sliding filament model of contraction. According to the model, the thick and thin filaments simply slide past each other. Figure 14-8 shows several positions in the shortening of the muscle, illustrating the sliding of the filaments. At the maximum length there is little or no overlap of the filaments (A), but as the muscle shortens there is more and more overlap until the fibers completely overlap (D). There is general agreement that the sliding filament model is an accurate description of what happens during muscle contraction.
| According to the sliding filament hypothesis, thick and thin filaments simply slide past each other to produce shortening. |
| Events leading to contraction |
|
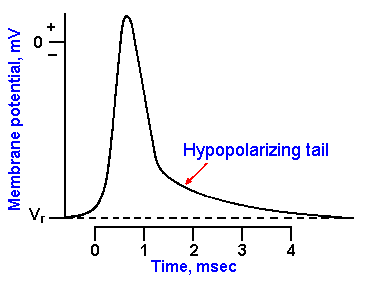
Fig. 14-9. The skeletal muscle action potential. The spike is followed by a depolarizing tail that lasts 4 to 5 msec. |
Although muscle contraction can be initiated by direct electrical stimulation of the muscle, it usually results from activity in the motoneurons innervating the muscle. An action potential initiated in an alpha-motoneuron propagates into the motoneuron terminals and releases acetylcholine into the synaptic cleft. The acetylcholine induces an end-plate potential in the muscle which, in normal muscle, always leads to an action potential in the muscle. The muscle spike is very much like the nerve spike but longer in duration and with a hypopolarizing tail on the falling phase that prolongs the spike by 3-4 msec. An example of a muscle spike is shown in Figure 14-9. The mechanism of generation of the spike in mammalian striated muscle is the same as that described for nerve in Chapter 3. The long (4-5 msec) hypopolarizing tail of the muscle action potential is probably the electrotonic reflection of the action potential as it conducts into the T tubules. At least, the tail disappears from the spike when the muscle is treated with glycerol and then returned to Ringer's solution, a treatment that more or less specifically ruptures T tubules, leaving the surface membrane and resting potential intact. The muscle still generates a spike but does not contract. Conduction of the spike into the T tubules is probably an active process as elsewhere on the membrane, and it is the hypopolarization of the T tubule that leads to contraction.
It is reasonable to ask why there exists such an elaborate system of tubules in striated muscle. The answer may lie in the synchronization of contraction of sarcomeres along the length of the muscle and in its depths. The myofibrils are located throughout the muscle fiber, but in mammalian muscle there is usually only one neuromuscular junction per fiber. If there were no way for the hypopolarization of the spike to get into the center of the fiber, the myofibrils on the surface would contract before those in the center. With the T-tubule system, the spike is conducted rapidly to all parts of the cell, reaching all of the myofibrils at nearly the same time. In the absence of such a mechanism, contracting segments of the myofibrils would stretch the non-contracting ones, lessening the force transmitted to the ends of the fibers and, therefore, to the joints.
The hypopolarization of the T tubules opens special voltage-gated channels in opposing regions of the sarcoplasmic reticulum membrane. By an as yet incompletely understood mechanism, this leads to a release of calcium from the cisternae of the sarcoplasmic reticulum into the region of the myofilaments. This is an essential step in the contraction mechanism; muscles depleted of calcium do not contract. The calcium diffuses to the thin filaments and binds to troponin. Each head of the myosin molecule (a molecule has two) is an ATPase, capable of hydrolyzing ATP to ADP and inorganic phosphate, releasing energy; however, according to current thought, tropomyosin inhibits the ATPase. The combination of troponin with Ca++ removes the tropomyosin inhibition, perhaps by inducing a conformational change in the thin filament.
| Cross-bridge theory |
|
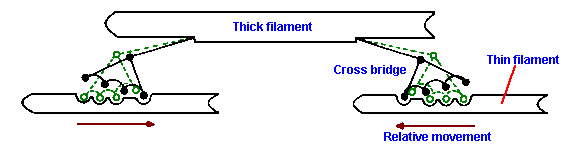
Fig. 14-10. Production of force according to the cross-bridge theory. Cross-bridges, projecting from the thick filament, are shown attached to the thin filament and swiveled (dashed outline). Directions of force and translation are indicated by arrows. (Nobel MIM, Pollack GH: Circ Res 40:33-342, 1977) |
To this point, most theories of contraction agree. There are, however, two plausible theories of how the force of contraction develops. The first, the cross-bridge theory, suggests that an actual physical binding of the myosin head to the thin filament occurs, that the hydrolysis of ATP causes a rotation of the head toward the tail, pulling on the compliant arm of the cross-bridge. This pull results in a relative movement of thin and thick filaments and tension and shortening of the sarcomere. In detail, ATP binds to myosin and then, in the presence of Ca++, troponin, and tropomyosin, a myosin binding site is exposed on the thin filament and a physical link is formed between actin and myosin. The ATPase activity of the myosin is then exerted, cleaving a phosphate bond of the ATP, releasing energy, and causing the myosin head to swivel. Figure 14-10 shows the way in which the myosin head is thought to rotate (solid and dashed outlines of myosin heads are meant to indicate two different positions of the heads as they rotate) and the relative movement of the filaments that results. Because the heads of the myosin molecules are oriented in opposite directions at opposite ends of the thick filament, each pulls its adjacent thin filament toward the center of the sarcomere and the sarcomere shortens.
When the hypopolarizing stimulus of the spike in the T tubules is over, calcium ceases to be
released by the cisternae of the sarcoplasmic reticulum and actively pumped into the longitudinal
portion of the reticulum. The Ca pump that pumps Ca from the cytosol back into the sarcoplasmic reticulum is an ATPase that is phosporylated and dephosphorylated during the pumping process. It pumps two Ca ions for each ATP hydrolyzed. In muscle, the Ca ATPase accounts for nearly 90% of the membrane protein and therefore is capable of pumping Ca ions rapidly. Typically, the cytosolic Ca concentration is restored to resting levels within 30 milliseconds. When calcium is removed from the myofibrils, ATP replaces ADP on the
myosin complex and the myosin-actin bond is broken. Because the muscle is elastic, it will be
restored to its resting length in the absence of a further stimulus to release calcium. Shortening is
an active process; lengthening is a passive process.
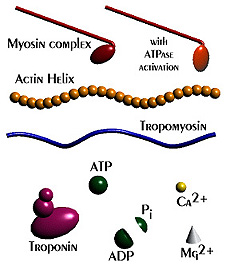
A single cycle of attachment, swivel, and detachment of the myosin head will produce a linear translation of the myofilaments of about 10 nm. If all cross-bridges in a myofibril cycle once synchronously, a relative movement equal to about 1% of the muscle length will occur, but obviously muscles shorten by more than 1%. The total shortening of a sarcomere during contraction may exceed 1,000 nm; therefore the relative movement of a thin and thick filament would be half this amount or 500 nm. To achieve this magnitude of change in total length when each cross-bridge cycle produces a 10-nm shortening, a minimum of 50 cycles must occur. The flexor muscles of the human upper arm can contract at the rate of 8 m/sec (Wilkie DR: J Physiol (Lond) 110:249-280, 1949), during which they can shorten by as much as 10 cm. This contraction rate gives a contraction rate for the sarcomere of 160 nm/msec. If a stroke of the cross-bridge is taken to be 10 nm, then at this rate there will be a minimum of 16 strokes/msec. Thus, the swivel time for the cross-bridge must be of the order of 60 sec. Calculations for the frog's sartorius muscle, which can shorten at up to 4 cm/sec, indicate a swivel time of about 1 msec, but this contraction occurs at a lower temperature than those in mammals. In any case, it is clear that the swiveling of the cross-bridge must be a fast mechanical process. At the right is an animation that shows the repeated nature of the process.
| The cross-bridge theory says that sliding is produced by physical attachment of myosin heads to actin and by rotation of the heads. |
The cross-bridge theory assumes that the force generated by the muscle is proportional to the number of cross-bridge linkages formed at that time and that the probability of formation of a cross-bridge is proportional to the speed of shortening, i.e., the probability is great when attachment sites move slowly past one another, small when they move rapidly. If tension is a function only of the number of cross-bridges, then there should be a linear relationship between length and tension such that tension increases with decreasing length because of the greater overlap of thick and thin filaments at shorter lengths. The force required to stretch the muscle at any time is therefore also proportional to the number of cross-bridges-it is the force required to break the actin-myosin bonds.
In summary, l) tension is developed by physical bonds between thick and thin filaments, 2) tension depends upon the degree of overlap between thick and thin filaments, 3) the cross-bridge originates at the thick filament and terminates at the thin filament.
- Tension is developed by physical bonds between thick and thin filaments.
- Tension depends upon the degree of overlap between thick and thin filaments.
- The cross-bridge originates at the thick filament and terminates at the thin filament.
Table 14-1
Summary of Events in Muscle Contraction and Relaxation
Synaptic transmission at neuromuscular junction
Action potential propagates along sarcolemma
Hypopolarization of T tubules
Ca++ released into sarcoplasm from sarcoplasmic reticulum
Ca++ bound by troponin
Cooperative configurational change in troponin and tropomyosin
Release of inhibition of myosin-ATPase
Link between thick and thin filaments, swivel of myosin head
Tension exerted
Shortening by sliding filament
Ca++ removed from sarcoplasm
Mg++ATP bound by actinomyosin
Cross-bridges disconnected
Actinomyosin-ATPase inhibited
Active tension disappears
Series elastic elements restore resting length
| Properties of contracting muscle |
When a muscle is stimulated either directly or synaptically, it develops tension and, if allowed to, it contracts, i.e., it shortens(3). In this section we will discuss the ways in which a muscle contracts.
|
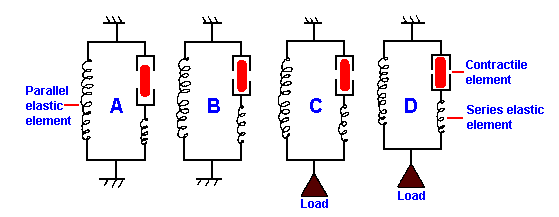
Fig. 14-11. Series and parallel elastic elements in muscle. A. Resting muscle contains elastic elements in series with the contractile elements (sarcomeres) and in parallel with them. B. During an isometric contraction, the muscle does not change length, but sarcomeres shorten, stretching the series elastic elements. C. During isotonic contraction, the contractile elements shorten, stretching the series elastic elelments, before they develop tension to lift the load. D. Muscle begins to shorten when contractile elements shorten further. |
Isometric versus isotonic contraction
When a muscle is stimulated after its ends or tendons have been fixed, it contracts but cannot shorten. This is called an isometric contraction (iso = same, metric = measurement or length). The muscle develops tension, but because it does not shorten, it does no external work (recall: work = force x distance moved). Careful observation reveals that during an isometric contraction some sarcomeres of the muscle shorten, stretching other sarcomeres and, in addition, stretching elastic elements of the muscle, increasing the tension measured at the tendon. Figure 14-11A is a schematic diagram of the muscle, showing elastic elements both in series and in parallel with the contractile elements (the myofibrils) of the muscle. When a whole resting muscle or a single resting muscle fiber is stretched, it opposes the stretch with a force that increases with increased stretch (like a rubber band). This elasticity is due to the parallel elastic elements that lie, for the most part, outside the contractile elements in elastic tissues, including tendons and the sarcolemma. (When the muscle contracts, the shortening of the muscle lags behind the shortening of the sarcomeres because of the series elastic elements.) Therefore, in the isometric contraction, the sarcomeres shorten and stretch the series elastic component even though the muscle as a whole does not shorten, as shown in Figure 14-11B. Even though there is no external work being done by the muscle, there is internal work being done.If only one end of the muscle is fixed, the muscle shortens and, if it shortens with a constant load, the contraction is isotonic (iso = same, tonic = tension). When the contractile elements shorten they must first stretch the series elastic elements and develop a tension equal to the load before the next increment in tension causes the load to be lifted. All of the contraction that occurs before the load is lifted is isometric. Even if the muscle carries no external load, it still must develop a tension equal to its own weight before it can shorten. When contractile forces exceed the load, shortening begins; tension remains slightly larger than the load throughout shortening. Shortening stops when active tension drops to the point where it equals the load. At this point, contraction again becomes isometric. The muscle lengthens (is stretched) when the total tension in the muscle falls below the load. Figure 14-11C and D show the changes in both series and parallel elastic elements and in the contractile elements during an isotonic contraction. In C, the contractile elements have shortened, stretching the series elastic elements, but the muscle has not shortened. In D, the further shortening of the contractile element leads to shortening of the muscle because the series elastic elements are already stretched.
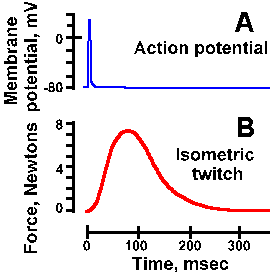
|
Fig. 14-12. Relation between muscle acton potential and twitch contraction. The time course of the action potential is indicated in A and the longer development of tension (ordinate) of the twitch contraction is shown in B. (Dudel J: Muscles. In Schmidt RF [ed]: Fundamentals of Neurophysiology, 2nd ed. New York, Springer-Verlag, 1978) |
Twitch and tetanic contractions
If a brief stimulus is applied to the muscle or a single stimulus is applied to the nerve, a single action potential will be elicited in the muscle and, after an activation delay of about 5 msec, the muscle will contract. The time-course of this contraction, called a twitch contraction, is shown in Figure 14-12B. The tension in the muscle rises rapidly to a maximum, about 50-80 msec after the stimulus and then returns to resting tension over the next 100-200 msec or so, depending upon the particular muscle. In A, the muscle action potential is reproduced for comparison with the twitch, which lasts about 100 times longer. The muscle whose contraction is shown in the figure, the adductor pollicis muscle of the thumb, is a fast muscle. As we shall see, mammals also have slow muscles that require 200 msec or more to attain their maximum twitch tension.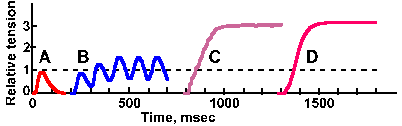
|
Fig. 14-13. Isometric contractions during repeated stimulation. A. Tension development during a single twitch contraction in a fast skeletal muscle. B. Summation of twitch contractions when stimulation is repeated at 10 per sec; note greater tension. C. Unfused tetanus evoked by stimulation at 50 per sec. D. Fused tetanus evoked by stimulation at 100 per sec; note maximum tension developed. (Vander AJ, Sherman JH, Luciano DS: Human Physiology: The Mechanisms of Body Function. New York, McGraw-Hill, 1970) |
A second stimulus, applied before the muscle has completely relaxed, induces another contraction that adds to the first, the sum of the tensions being greater than that of a single twitch. This event, as you probably have guessed, is called summation. An example is shown in Figure 14-13. A single stimulus evokes the twitch at the left in A. Repeated stimulation at a rate of 10/sec evokes the summed tensions in B. When the stimulus frequency is increased to 50/sec, the tension rises to a more-or-less steady value, much greater than the twitch tension. This summation, as in C, is called tetanus or tetanic contraction. Because individual twitch contributions to the tetanus can still be seen as bumps in the record, this tetanus is called unfused or incomplete. At even higher rates of stimulation, the maximum tension the muscle can sustain is obtained and there is no sign of individual twitches in the record. This is called fused or complete tetanus, and it is shown in Figure 14-13D for a 100/sec stimulation rate. Fast and slow muscles, as defined by their twitch times, also differ in the least stimulus frequency at which their contractions produce fused tetanus. Fast muscles may not fuse until stimulus rates equal or exceed 60/sec, whereas slow muscles may fuse at rates of only 16/sec.
| A single action potential produces a twitch contraction. Multiple contractions may sum. |
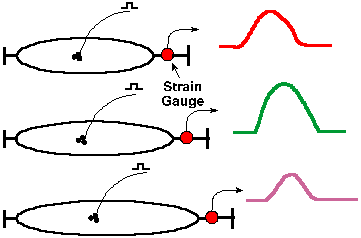
|
Fig. 14-14. Set up for length-tension experiments. The muscle is indicated by an ellipse with two tendons at either end of the long axis. A strain gauge has been introduced into one tendon to measure tension. The nerve to the muscle is indicated as is the pulse applied to initiate a twitch contraction. The middle drawing is meant to indicate resting length, the upper some length less than resting, and the lower some length less than resting. At the right of each drawing is the twitch contraction initiated in each case. |
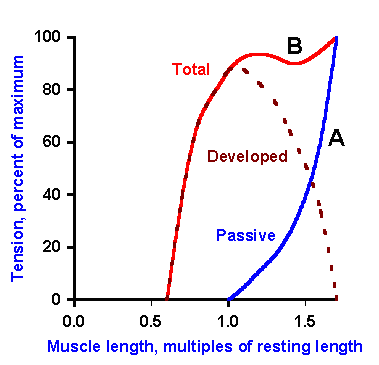
|
Fig. 14-15. Length-tension curve. Isometric tension (ordinate) is plotted against muscle length (abscissa) expressed as a fraction of resting muscle length, 1.0. The muscle's elasticity resists stretch, causing tension that follows the dotted curve; this is passive tension. When the muscle's length is fixed at a value on the abscissa and then it is stimulated to contract, it develops tension that lies on the dashed curve. This is the total tension or the sum of passive tension and developed tension. The difference (solid curve) between the dashed and dotted curves is the tension developed by the muscle when it contracts. This is maximum at the resting length. (Dudel J: Muscles. In Schmidt RF [ed]: Fundamentals of Neurophysiology, 2nd ed. New York, Springer-Verlag, 1978) |
Obviously, the maximum isometric contractile tension occurs when the muscle is at its resting length. At shorter or longer lengths, the isometric tension produced by the contractile elements is less. The exact shape of the curve for lengths longer than the resting length depends upon when and how the tension is measured (for a discussion see Noble MIM, Pollack GH: Molecular mechanisms of contraction. Circ Res 40:333-342, 1977). At lengths less than about 70% of resting length, the muscle develops no tension at all when stimulated. It follows that, during an isotonic contraction, a skeletal muscle can only shorten to about 70% of its resting length, and it can only develop tension at lengths between 70% and 180% of resting length. The isotonic length-tension curve approximately superimposes upon the isometric curve; hence during an isotonic contraction the muscle shortens to a length appropriate for the tension developed (i.e., to a length predictable from the isometric length-tension curve).
The length-tension curve can be explained by the cross-bridge theory. Instead of the length of the whole muscle, we could just as accurately have plotted sarcomere length on the abscissa of Figure 14-15, with resting length equal to about 2 µm. At resting length, the thin and thick filaments are in relative positions as shown in Figure 14-7C, with nearly the whole thick filament overlapped by thin filaments. In this position, all of the myosin heads are overlapped by the thin filament, and therefore all are available to form cross-bridges. Recall that in the cross-bridge theory the force of contraction is proportional to the number of cross-bridges formed. At longer lengths, such as those in A and B of Figure 14-7, some of the myosin heads are not overlapped by actin and therefore are not available to form cross-bridges. As a consequence, the tension developed will be less. At shorter lengths, such as D, the actin filaments from opposite ends of the sarcomere begin to interfere with each other and at shortest lengths the Z disc may block or otherwise impede movement of the thick filament.
| Skeletal muscles produce the maximum force when they contract from the resting length. |
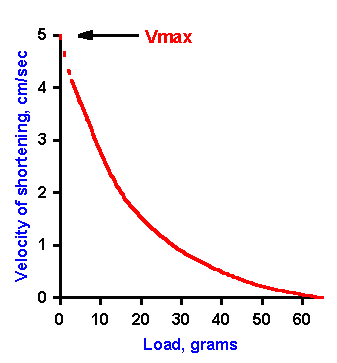
|
Fig. 14-16. Force-velocity curve. Velocity of shortening (ordinate) is plotted against the load (force) applied to the muscle (abscissa). As the load increases, the velocity of shortening decreases. The curve is extrapolated back to zero load, yielding the maximum velocity the muscle can achieve, Vmax. (Aidley DJ: The Physiology of Excitable Cells. Cambridge, Cambridge Univ Press, 1971) |
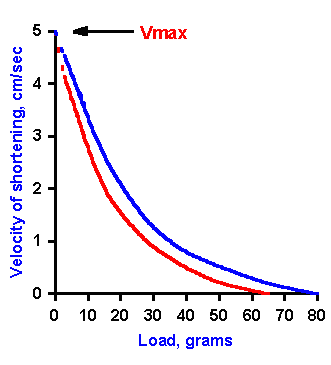
|
Fig. 14-17. Force-velocity curve at different initial lengths. Because the force developed varies with initial length, one would expect to find different force-velocity curves for different initial lengths. Two are shown in this figure. |
Force-velocity relation
Even though the conditions are right for an isotonic contraction, i.e., the muscle is fixed at only one end and a weight is attached to the other, the velocity of shortening will be zero (or negative, i.e., the muscle will lengthen) when the weight applied to the muscle is more than the muscle can lift. On the other hand, when there is no weight on the muscle, it will shorten at its maximum velocity. These are the boundary conditions of the force-velocity relation. Between these two extremes, the velocity of shortening decreases as the load increases. Earlier measurements of the velocity of shortening during isotonic contractions indicated that the velocity was an hyperbolic function of the load(4) being lifted, as shown in Figure 14-16. Newer measurements indicate that this is not usually the case (Hill AV: First and Last Experiments in Muscle Mechanics. London, Cambridge University Press, 1970). In both skeletal and cardiac muscle, the relationship, measured at the sarcomere level, is clearly not hyperbolic. The exact shape of the curve is, however, less important for this discussion than the fact that velocity decreases with increased load. Notice that the solid curve does not intersect the ordinate. This is because it is difficult experimentally to make the load zero in a practical way. (Under most conditions, the muscle must lift at least its own weight.) We can extrapolate the curve back to the ordinate to find out what the velocity at zero load would be (dashed line, extending the curve in Figure 14-16). This is the maximum velocity or Vmax.Actually, because the force exerted by the muscle is also related to its length, there will be a family of curves like that in Figure 14-16. The curve shown was obtained when the muscle started to contract at its resting length. The force generated at greater or lesser initial lengths will be less than that at the resting length. Therefore, all of the curves for lengths other than the resting length will be roughly parallel to, but below that shown in Figure 14-16. They will be roughly parallel except near zero load, where all the curves will converge onto the same value of Vmax (Figure 14-17).
| The smaller the load, the more rapid the contraction. |
The cross-bridge theory is able to account for the force-velocity curve by assuming that the rate constants for the processes of attachment and detachment of the cross-bridges are dependent upon the instantaneous position of the cross-bridge relative to the attachment site on the thin filament. The rate of attachment is zero after the cross-bridge passes the attachment site, but the rate of detachment is high. When approaching the attachment site, the cross-bridge cannot attach unless it is within a certain distance; beyond that distance, the rate of attachment is zero, but the rate of detachment is not. Detachment requires a certain minimum time. At low velocities, there is sufficient time for detachment, but at high velocities, there may not be. Thus, the cross-bridge may remain attached longer than it should and actually oppose the movement of the thin filament in the direction it has just propelled it. This will cause a force opposite in direction to that caused by sarcomere shortening and, therefore, reduce the effective contractile force. The greater the velocity, the greater the effect of the detachment failure.
| Mechanisms for grading force of contraction |
We have already discussed one mechanism for grading muscle force, that is, by grading the frequency of discharge in the motoneurons and thus the temporal summation of twitch contractions. The end-points of this continuum are, of course, a single twitch in the weakest element (the minimum) and a fused tetanic contraction in the strongest (the maximum single-unit twitch). Each muscle fiber is contacted by only one motoneuron, but a motoneuron can contact many muscle fibers. When a motoneuron discharges, it activates all of the muscle fibers with which it makes synaptic contact. A motoneuron and the muscle fibers it contacts are called a motor unit. A motor unit for muscles of the lower leg may contain as many as 1700 muscle fibers, whereas a motor unit for the extrinsic muscles of the eye may contain only 7 fibers. The fibers that make up a motor unit are not grouped together in a single bundle within the muscle, but are scattered in small bundles of a few fibers. Thus, forces produced by contraction of a single motor unit are distributed through the muscle. Because all of the muscle fibers of a motor unit contract simultaneously, it is more difficult to achieve fine gradations in the force of contraction when motor units are large. Suppose one motor unit contains 20 fibers and another only 5, and suppose each fiber can produce 1 gram of force. The minimum forces producible by the motor units would be 20 grams and 5 grams in a twitch contraction, and temporal summation would produce forces graded in 20- and 5-gram increments. When fine gradations are necessary, as they are in eye movements, the motor units are usually small.
The force of muscle contraction can also be varied by varying the number of motor units that are active, i.e., by spatial summation. Because the muscle fibers are connected in parallel and exert force on the same tendon, the forces produced in all the fibers will be summed at the tendon. Therefore, the greater the number of motor units active the greater the force on the tendon. Not all of the motor units within a muscle are the same size. As the force of contraction increases, larger and larger motor units are added to the contracting population. This, of course, means that the force of contraction will be a nonlinear function of the number of motor units.
With strong stimulation at rates higher than 5/sec, the force of contraction will increase over that of the twitches evoked by weak, lower frequency stimulation. This is the result of both spatial and temporal summation. The maximum tension a muscle can develop obviously occurs when all motor units produce fused tetanic contractions (absolute maximum). This occurs at stimulation frequencies over about 50/sec, well within the discharge rate capabilities of motoneurons. This forms the limits of control of muscle tension: a minimum of the tension produced by a single twitch in the smallest motor unit and a maximum of the tension of a fused tetanic contraction in all motor units. Nearly any force in between can be produced by some combination of contraction of different motor units and different motoneuron discharge rates, i.e., different amounts of spatial and temporal summation..
| A minimum tension is produced by a single twitch in the smallest motor unit and a maximum by the simultaneous fused tetanic contraction in all motor units. |
Tension develops smoothly during normal muscle contractions, not at all like the subtetanic contractions that we have seen. Yet, motoneurons have stable firing rates over a wide range of developed tensions, and they often fire at rates below those necessary to produce tetanic fusion. At these low frequencies, the motor units must behave in a twitchlike fashion. This means that the smoothness of development of tension is due to the asynchrony in contraction of different motor units.
| Fast and slow muscles |
There are two different types of skeletal muscles in vertebrates, the red and white muscles. Red muscles are red because they contain the protein myoglobin that, like hemoglobin, contains the iron-rich heme group. It is the heme group that gives both hemoglobin and myoglobin a red color and the ability to bind oxygen. White muscles are white because they contain little myoglobin. Red muscles are the more slowly contracting twitch muscles, the slow muscles, whereas white muscles are the more rapidly contracting fast muscles. As already noted, slow muscles also require a lower minimum rate of stimulation for tetanic fusion.
The concentration of myosin is about the same in both red and white muscle, but the concentration of myosin-ATPase is much higher in white muscle fibers. Red muscle contains many mitochondria and gets most of its ATP from oxidative phosphorylation. This source supplies ATP rapidly, and thus the red muscles are able to sustain contractions longer without fatiguing. In addition, red muscle is highly vascularized, receiving and using more oxygen than white muscle. This may also be a function of the high concentration of myoglobin. White muscle, on the other hand, contains few mitochondria and gets most of its ATP from glycolysis, the breakdown of glycogen (which occurs in high concentration in white muscle) into lactic acid. This source of ATP is not as efficient as oxidative phosphorylation, and therefore white muscles fatigue more rapidly than red muscles. White muscle is also more poorly vascularized. These deficiencies may not be a big problem because white muscles tend to be active for only short periods in normal behavior. Because of these differences, white muscles have been called "twitch now, pay later" muscles, whereas red muscles have been called "pay as you twitch" muscles.
Different muscles contain different types of muscle fibers. Some fibers contract rapidly, are glycolytic and fatigue rapidly. These are known as FG fibers, for Fast, Glycolytic. Other fibers are slowly contracting, oxidative, and slowly fatiguing. These are known as SO fibers, for Slow, Oxidative. Still other fibers contract rapidly and are both oxidative and glycolytic and are therefore known as FOG fibers. Although a motor unit consists of only one kind of muscle fiber, most muscles are mixtures of FG, SO and FOG fibers. The soleus muscle is a red muscle, and it contains almost exclusively SO fibers (87-100%, depending upon species), whereas the gastrocnemius, a white muscle, has a mixture of FG, FOG and SO fibers (41-66%, 14-38%, 5-45%, depending upon species).
| Although a motor unit consists of only one kind of muscle fiber, most muscles are mixtures of FG, SO and FOG fibers. |
The fibers in red and white muscles also receive different innervation. Fibers in red muscles are innervated by motoneurons of small diameter, thus lower conduction velocity, that discharge nearly continuously at low frequency. Fibers in white muscles receive innervation from larger motoneurons that have longer periods of silence between discharges, but discharge at high frequencies.
Table 14-2
Properties of White and Red Muscles
Property |
White muscles |
Red muscles |
| Twitch contraction time, msec | Fast, 50-80 | Slow, 100-200 |
| Minimum tetanic frequency | 60/sec | 16/sec |
| Myoglobin content | Low | High |
| Primary source of ATP | Glycolysis | Oxidative phosphorylation |
| Glycogen | High | Low |
| Myosin-ATPase activity | High | Low |
| Capillary blood flow | Low | High |
| Fatiguability | Easy | Difficult |
| Nerve fiber size | Large | Small |
| Nerve fiber activity | Intermittent, high frequency | Continuous, low frequency |
| Tension produced | Larger | Smaller |
The properties of both red and white muscles are summarized in Table 14-2. The properties of slow muscle fibers make them most suited to extended periods of contraction where a minimum force is required, e.g., in maintenance of posture. Fast muscle fibers are better suited to short periods of rapid contraction at higher forces, e.g., in sprint running. In fact, during exercise training there may be a differential effect on the two types of muscles. Strength training leads to hypertrophy of mainly white muscles with conversion of FOG to FG fibers. The number of fibers does not increase, but the size of fibers and the number of myofibrils do increase. This increases both the strength and velocity of contraction. Endurance training apparently affects mainly red muscle fibers, causing an increase in concentration of the enzymes of oxidative phosphorylation, an increase in the vascularization of the muscle and conversion of FG to FOG fibers, but no change in the ratio of fast to slow fibers and no change in muscle size.
| Electromyography |
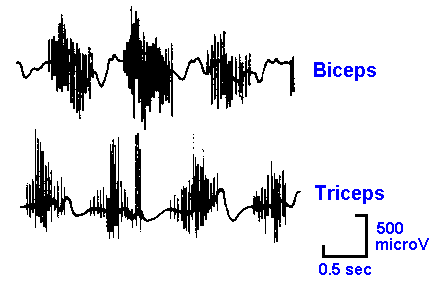
|
Fig. 14-18. Electromyographic records of activity in the human arm during alternate flexion and extension of the elbow. The upper trace shows the EMG from the biceps, and the lower trace, the EMG from the triceps muscle. (Ganong WF: Review of Medical Physiology, 7th ed. Los Altos, CA, Lange, 1975) |
When muscles contract, they generate action potentials. The action potentials result from transmembrane currents in muscle fibers that can be recorded extracellularly. This may be done in unanesthetized humans using small metal electrodes on the skin over the muscle or using hypodermic needle-electrodes inserted into the muscle. The record of the muscle contraction obtained in this way is the electromyogram, abbreviated EMG. When needle electrodes are used, it is often possible to detect the discharges in single muscle fibers near the electrode. Discharges in different fibers can sometimes be distinguished on the basis of the amplitudes of their spikes. Figure 14-18 shows the EMGs from human biceps (upper trace) and triceps muscles (lower trace) during alternate flexion and extension of the elbow. Note the spikes of different amplitudes and the general increase in spike density with each contraction.
| Cardiac muscle |
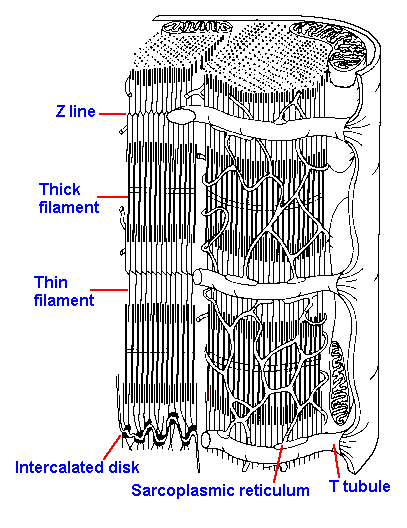
|
Fig. 14-19. Three-dimensional reconstruction of cardiac muscle, showing organization of myofibrils, T tubules, and sarcoplasmic reticula. Compare with Fig. 14-5. (Warwick R, Williams PL [ed]: Gray's Anatomy. 35th British ed, Philadelphia, WB Saunders, 1973) |
Structurally, cardiac muscle is similar to skeletal muscle in that it is striated, having both thick and thin filaments. It has a well-developed T tubule system, although the sarcoplasmic reticulum is not as large or as extensive as in skeletal muscle. Figure 14-19 shows the basic structure of cardiac muscle for comparison with Figure 14-5. Unlike those in skeletal muscle, the triads of cardiac muscle of humans are located at the Z line, giving only one per sarcomere. The mechanism of excitation-contraction coupling is the same as for skeletal muscle: The membrane action potential leads to an increase in Ca++ around the myofilaments that activates myosin-ATPase and leads to sliding of the thin and thick filaments. The source of the calcium is different in cardiac muscle. Because the sarcoplasmic reticulum is poorly developed, it cannot sequester the large amount of calcium that skeletal muscle can. Therefore, much of the calcium for contraction must come from extracellular sources; it comes in during the action potential.
There are a large number of different kinds of cells in cardiac muscle. These include cells of the sinoatrial node, the atrioventricular node, the atrium, the bundle of His, and the ventricle, each with a differently shaped action potential. The details of these differences are beyond the scope of this treatment. For our purposes, it is convenient to distinguish two kinds of cardiac muscle cells: pacemaker cells, like the Purkinje fibers, and contractile cells. Examples of a Purkinje fiber action potential (A) and a contractile cell action potential (B) are shown in Figure 14-20. Both action potentials are much longer in duration than spikes in nerve cells and skeletal muscle cells, 0.5 sec compared to 0.5 to 5.0 msec. The hypopolarizing phase of the Purkinje fiber's action potential is not different from that in skeletal muscle, and it appears to have the same ionic mechanism, i.e., a dramatic increase in sodium conductance. The rising phase of the contractile cell's action potential is shown in an expanded sweep in Figure 14-20C (right) along with that of the Purkinje fiber for comparison(5). The contractile cell's action potential has two rising phases, a rapidly rising phase, like that in the Purkinje fiber(6), and a more slowly rising phase. The fast phase has the same mechanism as the rising phase of Purkinje fiber action potentials, but the slower phase is the result of a slow inward current, carried mostly by Ca++. Calcium current activation occurs at a more hypopolarized level of the membrane potential than does sodium activation, and the inactivation of the calcium current is less rapid by about two orders of magnitude.
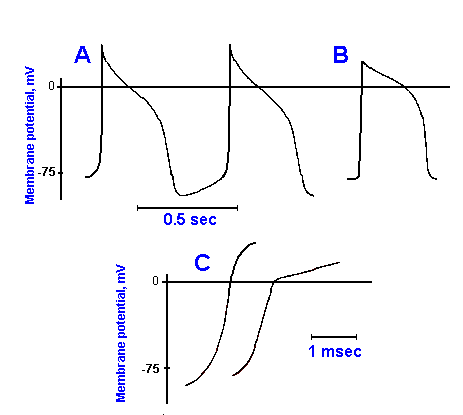
|
Fig. 14-20. Cardiac muscle action potentials. A. Two consecutive action potentials from a pacemaking Purkinje fiber. B. Action potential from a contractile fiber. C. The upstrokes of Purkinje fiber and contractile fiber action potentials shown in expanded sweeps. |
The long plateau of the action potential in cardiac muscle serves two functions: It provides a more prolonged contraction without resorting to tetanus, and it provides a longer refractory period to prevent the heart from contracting prematurely. This plateau is produced by a number of factors, the most important of which is a decrease in potassium conductance with hypopolarization, followed by a slowly developing increase that brings the potassium conductance to a final value just slightly greater than resting levels in Purkinje fibers and to resting levels in contractile cells in about 300 msec. A change in membrane conductance with changes in membrane potential is called rectification by biophysicists. This change in potassium conductance is called anomalous rectification.
Changes in sodium and potassium conductances are shown in Figure 14-21B on the same time scale as the Purkinje fiber action potential in A. During the plateau of the action potential, membrane resistance is high. Thus, after the peak of the action potential, gNa+ begins to decrease, albeit more slowly than in skeletal muscle and nerve, and in contractile cells the slow inward current persists. In addition, there may be a small outward current due to chloride ions(7). These currents would be effectively canceled by a big outward K+ current if the potassium conductance remained normal, but, because gK+ is depressed due to anomalous rectification, the sum of the outward iK+ and outward iCl- is just slightly larger than inward sum iNa+ + iCa++, and the membrane repolarizes only very slowly. However, as gK+ increases during the spike (because the membrane is slowly repolarizing), iK+ increases while gNa+ and gCa++ are decreasing, and the membrane begins to repolarize faster and faster (the downstroke of the action potential) until the resting membrane potential, Vr , is reached.
| In pacemaker cells, the membrane potential is almost always changing; so there is no true resting potential. |
In Purkinje fibers, the membrane really has no Vr because the membrane potential is constantly changing. This is a property of pacemaker cells, cells that have their own intrinsic rhythms of activity. As soon as one action potential is completed, the membrane immediately begins to generate another, even in the absence of any neural connections. This kind of behavior is never found in skeletal muscle; the muscle does not contract in the absence of innervation. When the Purkinje fiber membrane repolarizes, it returns to the maximum negative diastolic membrane potential, Vd, where membrane current is zero, im=0, but it stays there only for an instant before the membrane begins to hypopolarize again. At Vd, the membrane current is zero because the outward potassium current is exactly balanced by inward sodium, calcium and chloride currents. The membrane begins to hypopolarize because the potassium conductance, after its initial decrease and slow rise to a level just higher than resting level, begins to decrease toward resting level. As it does so, the potassium current decreases and a point is reached where iK+ no longer balances the inward currents, and the net inward current hypopolarizes the membrane. When the critical firing level is reached, the action potential is initiated. The pacemaker rhythm that develops is myogenic (of muscle origin) rather than neurogenic (of neural origin), but it can be influenced by neurotransmitters; these decrease or increase the rate of formation of action potentials by decreasing or increasing the rate of hypopolarization after the action potential. With increased rate, the succeeding action potential begins sooner; with decreased rate, it begins later.
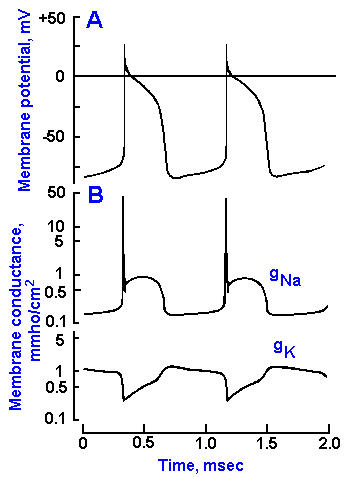
|
Fig. 14-21. A scheme that explains the form of the Purkinje fiber action potential and the pacemaking activity. Two consecutive Purkinje fiber action potentials are shown in A. Changes in sodium and potassium conductances during the action potential are shown in B. The ordinates are membrane potential (A) and membrane conductance (B); abscissa is time. (Noble D: J Physiol (Lond) 160:317-352, 1962) |
Not all muscle cells are pacemaker cells, but the whole heart must behave as a unit for it to be an effective pump. Contractions are partly synchronized by electrotonic spread of action potentials from one cell to another. Heart muscle is a network of branching muscle fibers connected to each other by gap junctions that are strung together in a structure called the intercalated disk. An intercalated disk is shown in Figure 14-19. The gap junction is thought to be a low impedance pathway, much like an electrotonic synapse. It seems likely that transmission of the action potential from cell-to-cell in cardiac muscle is the same as transmission from cell-to-cell at an electrotonic synapse.
Cardiac muscle behaves much like skeletal muscle, but it exerts a passive tension when
stretched at much shorter lengths. In fact, when the muscle is stretched from a length even
shorter than resting length, there is a resistance to the stretch. In other words, cardiac muscle
experiences elastic tension even at resting length (skeletal muscle does not). In addition, the maximum developed tension in cardiac muscle occurs, not at the
resting length, but when it is stretched beyond resting length (Figure 14-22). The result is that when more blood
returns to the heart from the veins, the muscle fibers of the heart will be stretched more, and the
blood will automatically be pumped out more forcefully than when the heart is just normally full.
This is the basis of the Frank-Starling mechanism in the heart.
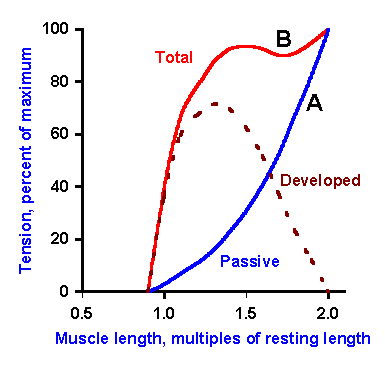
|
Fig. 14-22. Length-tension curve for cardiac muscle. See legend for Fig. 14-15 for details. Note that the maximum force is not developed at the resting length but at a length longer than the resting length. |
The force-velocity curve for cardiac muscle has the same shape as that for skeletal muscle and similar parallel curves are generated for different initial lengths, with a constant Vmax. Increasing the amount of blood in the heart, therefore, does not increase the contractile ability or contractility of the muscle. On the other hand, the actions of certain agents like norepinephrine or circulating hormones include increasing Vmax or contractility of cardiac muscle.
| Cardiac muscle differs from skeletal muscle in its structure, in the source of its calcium and in the fact that it can develop tension at lengths less than resting length. |
| Smooth muscle |
There is considerable diversity among smooth muscles, but all share a lack of the cross-striations characteristic of cardiac and skeletal muscle and an innervation by fibers of the autonomic nervous system like cardiac muscle. This is unlike skeletal muscle that is innervated by fibers of the somatic system. Smooth muscle fibers are smaller than skeletal muscle fibers and are filled with filaments oriented approximately along the long axis of the fiber. There are both thick, myosin-containing filaments and thin, actin-containing filaments, but they are not interdigitated like those in striated muscle. The thin filaments appear to be attached to the plasma membrane or to some structure in the cytoplasm. In general, there is twice as much actin but only one-third as much myosin in smooth muscle as in striated muscle.
Smooth muscles develop tension that varies with muscle length in a manner similar to that in skeletal muscle, but over a much wider range of muscle lengths, nearly twice that of skeletal muscles. This property is appropriate for their functions as linings of hollow organs; even when the organ is greatly distended, smooth muscles can still exert considerable tension. Many people assume that the presence of a length-tension curve implies that contraction occurs by a sliding filament mechanism, but how this occurs is not known. It has been shown that substantial tension can be developed in myofibrils to which myosin heads have been added in the presence of Ca++ and ATP, but in which thick filaments are absent (Oplatka A, Gadasi H, Borejdo J: Biochem Biophys Res Comm 58:905-912, 1974). This could be related to the contraction mechanism in smooth muscle.
Stretching a denervated smooth muscle causes it to actively contract, a phenomenon never seen in skeletal muscle. Presumably, stretching, like the occurrence of an action potential, causes an increase in cytoplasmic Ca++ that comes either from the sarcoplasmic reticulum or directly across the cell membrane. Smooth muscle contraction is much slower than that of skeletal muscle. This may be due to slow diffusion of Ca++ from outside the cell or to the slow rate of ATP hydrolysis or both.
Smooth muscle, like cardiac muscle, undergoes spontaneous, rhythmic contraction, driven by activity in certain pacemaker cells that behave like pacemaker cells in cardiac muscle. This pacemaker activity is conducted through gap junctions to neighboring smooth muscle fibers that do not generate pacemaker activity. This is typical of a type of smooth muscle called single-unit smooth muscle. Contractile activity of single-unit smooth muscle can be altered by nerve activity or hormones. This kind of smooth muscle also contracts in response to rapid stretching. Single-unit smooth muscles are found in intestinal tract, uterus, and blood vessels.
Multi-unit smooth muscles are found in the lungs, arteries, and erectile tissues of hair follicles. These smooth muscles contain few gap junctions, and therefore contractions do not spread from cell-to-cell as in single-unit smooth muscle. Multi-unit smooth muscle is richly innervated, with each cell receiving innervation from more than one nerve fiber and each fiber innervating several cells. Like skeletal muscle, multi-unit smooth muscle has motor units; unlike skeletal muscle, the neural inputs to these smooth muscles can be either excitatory or inhibitory. The response of the whole muscle depends upon the number of motor units active, the frequency of discharge in the fibers and the relative amount of excitatory and inhibitory input. Multi-unit smooth muscle activity can be initiated by hormones, but it is not much affected by rapidly stretching the muscle.
| Summary |
Under normal circumstances, contraction of skeletal muscle is initiated by action potentials in motoneurons which arrive at the neuromuscular junction and cause the release of acetylcholine from their terminals. The acetylcholine produces in the muscle, a hypopolarizing postsynaptic potential, the end-plate potential, which always initiates an action potential in the normal muscle fiber with normal innervation. The muscle action potential sweeps down the muscle membrane into the T tubules and somehow causes release of calcium from the cisternae of the sarcoplasmic reticulum. Calcium binds to troponin, and there is a release of inhibition of myosin ATPase, hydrolysis of ATP, and relative translation (sliding) of thick and thin filaments, causing the sarcomere to shorten and tension in the muscle to increase and perhaps causing the muscle to shorten. It is thought that actual linkages are formed between thick and thin filaments (cross-bridges), and the cross-bridges rotate. Contractions are terminated by removal of calcium from the sarcoplasm into the thin longitudinal tubules of the sarcoplasmic reticulum. The cross-bridge theory maintains that relaxation occurs when the cross-bridges are disconnected, the series elastic elements then restore the muscle to resting length.
Muscle contraction can be isometric or isotonic in the experimental situation. Isometric contractions are those where tension develops in the muscle, but it does not change length. Isotonic contractions are those in which the muscle experiences a constant tension, but may shorten. During movement, muscle contraction is probably a mixture of contractions that are isotonic, isometric, and neither, with both length and tension varying.
A single action potential in a motoneuron will initiate a short, twitch contraction in the muscle that it innervates. If several action potentials arrive at the muscle close together in time, the twitches may sum. Summation may result in a sustained contraction that is known as tetanus.
Striated muscles develop their maximum isometric tensions at their resting lengths and develop only smaller tensions at lengths greater or less than resting length. The velocity of shortening of a muscle depends upon its load, the greater the load, the lower the velocity. Expressed another way, the greater the velocity of shortening, the smaller is the load that can be lifted by the muscle.
The force of muscle contraction can be graded by changing the frequency of discharge in active motor units and by changing the number of active motor units, resulting in forces graded between the tension of a twitch in the smallest motor unit to the tension of a fused tetanus in all motor units of the muscle.
Fast muscle is distinct from slow muscle in its faster twitch contractions, higher maximum tetanic frequencies, lower myoglobin content, lower blood flow, easier fatiguability, and innervation by larger axons that discharge intermittently at higher frequency. A motor unit contains either fast or slow muscle fibers, but a muscle is usually a mixture of both fast and slow fibers.
The EMG is a recording of the action potentials of groups of muscle fibers that lie near the recording electrodes. If needle electrodes are used to record the EMG, the discharges of single fibers can often be distinguished.
Cardiac muscle differs from skeletal muscle in the shape of its action potentials. All action potentials in cardiac muscle are more slowly developing (the hypopolarization phase is longer) and of longer duration. These differences are due, at least in part, to the slow inward calcium current and perhaps different channel activation kinetics and to anomalous rectification, respectively. Cardiac muscle also shows rhythmic activity that is myogenic, never seen in skeletal muscle. Also unlike skeletal muscle, cardiac muscle experiences elastic tension even at resting length and is able to develop tension at lengths shorter than resting length.
Smooth muscle behaves either as a single unit, i.e., there are many gap junctions causing the whole muscle to contract more-or-less at once, or as multiple units, i.e., there are few gap junctions, but rich innervation, each cell capable of independent contraction. In single-unit smooth muscle, rhythmic contractions are myogenic; in multi-unit smooth muscle they are neurogenic. Single-unit smooth muscle contracts in response to rapid stretch; multi-unit smooth muscle does not.
| Suggested Reading |
- Ariano MA, Armstrong RB, Edgerton VR: Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21:51-55, 1973.
- Bendall JR: Muscles, Molecules and Movement. New York, American Elsevier, 1970.
- Carlson FD, Wilkie DR: Muscle Physiology. Englewood Cliffs, NJ, Prentice-Hall, 1974.
- Ebashi S, Endo M, Ohtsuki I: Control of muscle contraction, Q Rev Biophys 2:351-384, 1969.
- Gordon AM, Huxley AF, Julian FJ: Tension development in highly stretched vertebrate muscle fibres. J Physiol (Lond) 184:143-169, 1966.
- Gordon AM, Huxley AF, Julian FJ: The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol (Lond) 184:170-192, 1966.
- Hill AV: First and Last Experiments in Muscle Mechanics. London, Cambridge Univ. Press, 1970.
- Huxley AF, Simmons RM: Proposed mechanism of force generation in striated muscle. Nature 233:533-538, 1971.
- Huxley AF: Muscular contraction. J Physiol (Lond) 243:1-43, 1974.
- Julian FJ, Moss RL, Solens MR: The mechanism for vertebrate striated muscle contraction. Circ Res 42:2-14, 1978.
- Noble MIM, Pollack GH: Molecular mechanisms of contraction. Circ Res 40:333-342, 1977.
- Oplatka A, Gadasi H, Borejdo J: The contraction of "ghost" myofibrils and glycerinated muscle fibers irrigated with heavy meromyosin subfragment-1. Biochem Biophys Res Comm 58: 905-912, 1974.
- Wilkie DR: The relation between force and velocity in human muscle. J Physiol (Lond) 110: 249-280, 1949.
- Zierler KL: Mechanism of muscle contraction and its energetics. In: Mountcastle VB [ed]: Medical Physiology. 13th ed. Vol. 1, St. Louis, Mosby 1974.
Footnotes:
1. In Fig. 14-1, muscle fibers are rectangles or parallelograms, tendons are lines radiating from the muscle fibers or vertical lines extending from the two sides of muscle fibers, arrows labeled f indicate direction of force exerted by fibers, and arrows labeled F indicate direction of force exerted by the whole muscle.
2. Actually, it is generally accepted that in Limulus, the horseshoe crab, the A bands do change length when contractions occur at lengths less than the resting length. They apparently do not in mammalian muscle.
3. An active muscle always develops tension but does not always shorten.
4. Another word for force is load.
5. The differences are exaggerated in this figure for didactic purposes.
6. Do not confuse the Purkinje fibers (muscle) of the heart with Purkinje cells (nerve) of the cerebellum.
7. The chloride current is outward because the driving force at the plateau is outward (the membrane potential is positive outside); chloride ions actually enter the cell.
[TOC] [Chapter 15] [Glossary] [Index] [Abbreviations]