
 Chapter 5
Chapter 5 
SOMESTHESIA: PERIPHERAL MECHANISMS
The broadest definition of somesthesia is the awareness of having a body and the ability to sense the contact it has with its surroundings. Our concerns at this point are about the receptors that serve to make us conscious of our bodies. Receptors are generally put into two broad classes: the exteroceptors, that sense stimuli from outside the body and signal what is happening in the outside world, and the enteroceptors, that receive stimuli from inside the body and tell us what is happening in the inside world. The broad class of exteroceptors includes, in addition to receptors in the skin, receptors for light in the eye, sound in the ear, and for chemical substances in the nasal mucosa and tongue. These specialized receptors are discussed in future chapters; for now, we will concentrate on the skin.
| The Exteroceptors |
The skin serves many functions:
- as protection from injury and dehydration
- as a radiation surface and regulator in temperature maintenance
- in secretion of chemical substances, such as pheromones that function as attractants or repellents
- as camouflage due to coloration in some species
- in reception of mechanical, thermal and, to some extent, chemical stimulation
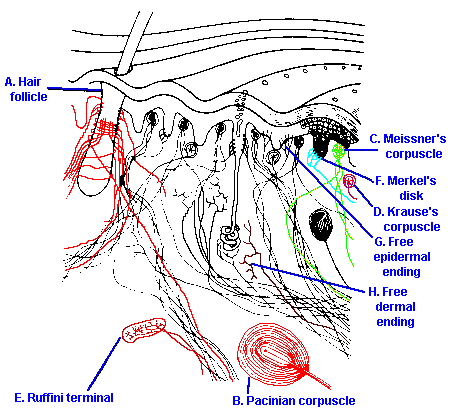 |
Fig. 5-1. A section through a trasitional region between glabrous and hairy skin showing the locations and arrangements of various dermal and epidermal receptors (Warwick R and Williams PL [ed]: Gray's Anatomy, 35th ed. Philadelphia, WB Saunders, 1973). |
From our present point of view, we may think of the skin as a sheet of sensory receptors held together and supported by a network of connective tissue and blood vessels. Figure 5-1 shows a cross section through a transitional region between glabrous and hairy skin. The outer layer or epidermis is composed of four to five layers of cells and connective tissue and is devoid of blood vessels. The epidermis receives its nutrients from the dermis immediately beneath it. The dermis consists mainly of loose connective tissue. Nerve fibers course into the skin through the dermis, and many of them end at the dermal-epidermal border where many of the sensory receptor structures are located. Figure 5-1 shows several of the types of receptors that are typical of skin. Structure A is a typical hair follicle-note that all hairs are innervated and thus serve as sensory receptors. The nerve fibers associated with a hair enter the follicle and follow a wandering course up and down along the root sheath and also around it. This winding pattern of the nerve fiber may determine how the receptor responds to hair movement, but as yet we do not know how. In addition to hair follicles, there are many encapsulated nerve endings found at the dermal-epidermal border. These are endings surrounded by specialized structures; a few of the types are shown in the figure (B-F). These structures vary somewhat in form so that it is not always clear in which class a particular structure belongs. The largest class of receptors is that with no specialized structure at all, the free nerve endings (G). Near their termination, the nerve fibers simply branch many times, and the many tiny terminal "twigs" lie in the dermis, near the border between the dermis and epidermis, or sometimes penetrate into the epidermis itself.
Many attempts have been made to associate different receptor structures with particular sensations, but there appears to be no clear relationship between structure and sensation. One problem is that the sensations associated with skin are surprisingly complex. Nearly everyone allows that there are (1) mechanical sensations, (2) thermal sensations, and (3) nociceptive or pain sensations, but only some will divide mechanical sensations into touch, pressure, and pinch, whereas others maintain that the list should also include vibration, tickle, itch, and perhaps others. Clearly, we may have more describable sensations than we have receptor types to account for them.
The problem is further compounded if we realize that we experience all of the normal skin sensations on the pinna or auricle, the external part of the ear, yet the pinna probably has only free nerve endings. Similarly, the cornea of the eye can sense temperature and pain, but has only free nerve endings. Although there is not a one-to-one relationship between receptor structure and sensation, that is not to say that there is no relationship at all. Free nerve endings are usually associated with the sensations of pain, temperature, and what many call crude touch, a sensation that requires firm pressure to elicit and is difficult to localize(1). The encapsulated endings are associated with light touch and pressure when they lie superficially within the skin and with deep pressure and tissue deformation when they lie deep within the tissue. Hair receptors, of course, can be associated with a class of sensations that accompany hair movement; these sensations have no special terminology.
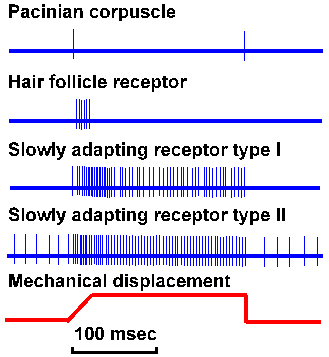 |
Fig. 5-2. Responses of cutaneous primary afferent fibers. A mechanical indentation or displacement was applied to four different types of receptors, the monitor of the movement being shown in trace 5 (numbered from the top down). The action potential responses of two rapidly adapting fibers are shown in traces 1 and 2: They respond only at the onset or offset of the stimulus. Responses of the two slowly adapting fibers are shown in traces 3 and 4: They discharge throughout the stimulus. A 100-msec time base is shown in trace 6. |
Mechanical sensations
If recordings are made from the sensory nerve fibers innervating particular cutaneous receptors, the stimuli that best excite each type of receptor, the adequate stimuli, can be identified. Examples of recordings from four different primary afferent fibers serving four different kinds of receptors are shown in Figure 5-2. A monitor of the mechanical displacement of the structure is shown in trace 5-the probe indented the receptors in traces 1, 3 and 4, and pushed the hair laterally in trace 2. Traces 1 to 4 show the spike discharges recorded from the fibers, the primary afferent fibers. The bottom trace is a time scale, with each division representing 100 msec. The discharge pattern of the Pacinian corpuscle should already be familiar. The fiber discharges when the receptor is compressed and again when the receptor is restored to its resting state-the discharge is rapidly adapting or phasic. The same kind of discharge pattern is seen in recordings from afferent fibers associated with hairs when a hair (trace 2) is displaced. The hair receptors are all rapidly adapting, i.e., they are incapable of signaling sustained stimulation. On the other hand, the slowly adapting receptors, types I and II(2), can signal the presence of a sustained stimulus. They begin discharging with the indentation and continue to discharge until the stimulus is removed. The type I receptor is a receptor associated with a Merkel's disk, whereas the type II is associated with a Ruffini ending.There are a number of characteristics of a mechanical stimulus, that are distinguished by physiologists, about which the central nervous system might need to be informed. First, there is simple stimulus detection-is there a stimulus present or is there not? All of the receptors just listed are exquisitely sensitive to mechanical stimuli, requiring displacements of only a few to tens of micrometers to excite them. Some require the stimulus actually to contact the skin, for example, type I and type II and Pacinian corpuscles, and some do not, such as hair receptors. The latter class may serve a distance receptor function, allowing stimuli to be detected before they contact the skin.
Every mechanical stimulus causes a displacement of a receptor when it excites one-hairs are
moved, skin is indented-and it might be of some value to know by how much the receptor was
displaced. A receptor must discharge when the stimulus is stationary, that is, when the receptor is
being held at a constant displacement, in order to signal the magnitude of the displacement.
Clearly, Pacinian corpuscles and hair receptors cannot signal displacement magnitude because
they discharge only when the stimulus is actually moving. That is not to say that the displacement
cannot be derived from their discharge. As we shall see, the hair receptors are good detectors of
stimulus velocity; all that is required is a relatively accurate internal clock to derive the
displacement from the velocity:
![]() velocity = displacement/time or displacement = velocity x time
velocity = displacement/time or displacement = velocity x time
However, displacement magnitude information is not directly encoded in the discharges of rapidly adapting receptors. An example of a receptor that does signal displacement is shown in Figure 4-8. The responses shown are for a Type I slowly adapting receptor; Type II slowly adapting receptors also signal displacement magnitude.
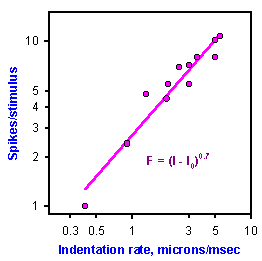 |
Fig. 5-3. Response of a velocity detector. The relationship between rate of indentation (abscissa) and number of impulses evoked per stimulus (ordinate) in a double logarithmic plot is shown. The threshold stimulus, 1.6 microns/msec, has beeen subtracted from abscissa values. To find actual stimulus velocity add 1.6 to values on the abscissa (Schmidt RF [ed]: Fundamentals of Sensory Physiology. New York, Springer-Verlag, 1978). |
It might also be useful to know the velocity of a mechanical stimulus, i.e., the rate of change of displacement or force. In order to signal velocity, a receptor must either increase or decrease its rate of discharge with increases in stimulus velocity. An example of this behavior is shown for a Meissner's corpuscle in glabrous skin in Figure 5-3 where receptor discharge frequency is plotted on the ordinate against indentation velocity on the abscissa. The relationship between these two variables is clearly nonlinear (this is a log-log plot), although the velocity of the stimulus is encoded in the discharge frequency(3). Hair receptors also encode the velocity of hair displacement by increasing frequency with increasing velocity. It is normally assumed that a receptor that signals displacement magnitude cannot also signal velocity; however, there is no evidence to support this assumption. Slowly adapting receptors, both types I and II, encode displacement velocity in their discharge frequency, and they must be considered as candidates for velocity receptors.
Another feature of a stimulus that might be detected is its frequency of application. Regular, periodically recurring stimuli induce a sensation of vibration or flutter. The Pacinian corpuscle discharges one spike for each displacement over a broad range of displacements and velocities and cannot supply information about either displacement magnitude or velocity. However, it discharges an action potential for each cycle of the vibratory stimulus up to 600 Hz and is normally considered to be a vibration receptor. Again, receptors that encode other features of the stimulus are considered not to be receptors for vibration, but most cutaneous receptors are capable of responding to vibratory stimuli at frequencies of 500 to 600 Hz at least for a short time (Burgess PR, Petit D, Warren RM: J Neurophysiol 31: 833-848, 1968). Human vibration perception is clearly within the ability of these receptors.
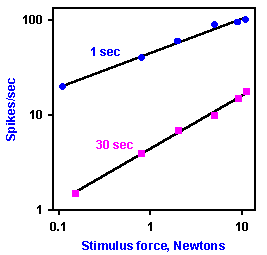 |
Fig. 5-4. Response of a pressure receptor to various constant-force stimuli. The relationship between stimulus force(abscissa) and discharge frequency (ordinate) at 1 and 30 sec after the beginning of a 40-sec duration stimulus. Threshold stimulus values at each time during the maintained stimulus were subtracted from applied intensities. Each point is the average of 10 measurements (Schmidt RF [ed]:Fundamentals of Sensory Physiology. New York, Springer-Verlag, 1978). |
Pressure receptors should signal stimulus pressure in their
discharge frequency. An example of a receptor that does this is
shown in Figure 5-4, which is again a log-log plot. Threshold
stimulus values at each time during the maintained stimulus were
subtracted from applied intensities. Each point is the average of 10
measurements. The slowly adapting receptor whose discharge is
shown in Figure 5-4 clearly signals increasing force of the stimulus
applied to it by increasing its discharge frequency. The increasing
force will usually result in increased displacement as well, and it is not
known whether any receptor signals force or displacement
independently. Types I and II slowly adapting receptors are capable
of signaling pressure as well as the duration of a mechanical stimulus.
 |
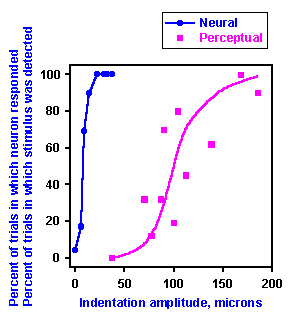 |
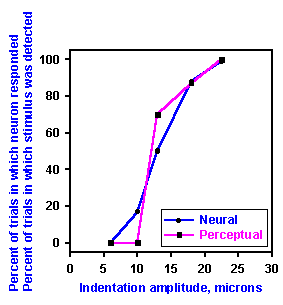
Fig. 5-5. Neural and perceptual thresholds for the same test point on the skin. The stimuli were delivered to a point on the skin that yielded the lowest threshold response from an axon assoociated with rapidly adapting receptors. The amount of indentation (abscissa) is plotted against the percent of stimulus presentations in which the stimulus was detected and against the percent of the stimulus presentations in which the axon discharged (ordinate). Data are shown for a stimulus point on the distal phalanx of the thumb (upper graph) and a point on the palm of the hand (lower graph). Neural data are indicated by open circles, perceptual data, by filled circles (Vallbo AB and Johansson R: Skin mechanoreceptors in the human hand: Neural and psychophysical thresholds. In Zotterman Y [ed}: Sensory Functions of the Skin in Primates, with Special Reference to Man. Oxford, Pergamon Press, 1976). |
In summary, there are obviously candidate receptors to signal stimulus presence, displacement, velocity, force, frequency, and duration. At this point, we can only say that the central nervous system receives this information from particular receptors; we cannot say that the nervous system actually uses the information. There have been a number of attempts to correlate psychophysical measurements in humans with the discharges of particular receptors. Initially, the receptor behavior was studied in animals, but more recently the behavior of human receptors has been observed. Two examples of the results of these experiments are shown in Figure 5-5. In the upper graph are shown nerve discharge frequencies and detection thresholds for indentations of the distal phalanx of the thumb. Filled circles indicate the percentage of the presentations of indentations of a given amplitude in which the stimulus was detected by the human subject, whereas open circles indicate the percentage of indentations at that amplitude that evoked a discharge from the receptors. There is clearly a close coherence between these two plots, but such a close fit is not always found. The thresholds for similar receptors on the palm of the hand are considerably lower than detection thresholds at the same sites (Fig. 5-5, lower graph). These data are typical of the results in experiments to date. Sometimes there is a close correlation between psychophysical results and neural results, and sometimes there is not. Even when there is a close correlation, we cannot be sure there is a causal relationship, i.e., that the neural discharge causes the sensation.
| A receptive field is the area of skin over which the application of a stimulus excites a primary afferent fiber. |
Receptive fields
The area of skin over which the application of a stimulus excites a given primary afferent fiber is called the receptive field of that fiber. As far as we know, a primary afferent neuron only innervates one particular type of receptor, though it may innervate a number of individual receptors of that type. For example, a hair afferent neuron may innervate anywhere from a few to 100 hairs and a given hair may receive innervation from 2 to 20 different fibers. Thus, there is considerable overlap in the receptive fields of different fibers. The size of a receptive field varies over the body surface, with those located on the extremities being the smallest, of the order of a few square millimeters on the digits, growing in size along the leg or arm, and reaching a maximum size on the trunk. This arrangement might account, in part, for the observed distribution in two-point thresholds, a commonly used measure of touch sensitivity. Two-point thresholds can be tested by using an ordinary pair of dividers(4). When the closed dividers are touched to the skin, the perception is of being touched with only a single point. As the dividers are opened more and more on successive applications to the skin, a separation of the points is reached at which the perception is of being touched with two points. The separation at which this first happens is the two-point threshold. Plotted in Figure 5-6 are the two-point thresholds expressed in millimeters on the abscissa against the position on the body on the ordinate. The distribution of two-point thresholds is much like the distribution of receptive field sizes described above. A moment's reflection will show why this should be so. In order for two sensations to be evoked, the stimuli must activate at least two primary afferent fibers. (This comes about because two action potentials cannot occupy the same region of membrane at the same time.) At minimum two-point distances, this is more likely in areas of skin with smaller receptive fields than in areas with larger ones.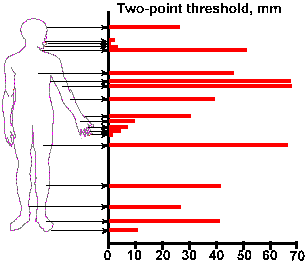 |
Fig. 5-6. Distribution of two-point thresholds over the surface of the body. The threshold is plotted as the distance between two points that just yields a sensation of two pints instead of one (Ruch TC: Somatic sensations. In Ruch TC and Patton HD [ed]: Physiology and Biophysics, 19th Ed., Philadelphia, WB Saunders, 1965). |
Receptors are discrete structures embedded in or residing just under the skin. Although in
some regions the density of receptors is very high, there are always areas in which there are no
receptors. Because of their extreme sensitivity to mechanical stimulation and the spread of
mechanical disturbances over the skin, receptors may nevertheless appear to be distributed
continuously. Table 5-1 shows the density of touch, pain and temperature spots (presumably these
correspond to receptor locations) in various regions of the skin. The data have been arranged in
descending order of touch spot density. On the glabrous surface of the hand and the nose, touch
spots are very dense; in hairy skin, the density is much lower.
Table 5-1
Sensitive Spots Per Square Centimetera | ||||
Touchb |
Pain |
Cold |
Warmth | |
| Ball of thumb | 120 | 60 | ||
| Tip of nose | 100 | 44 | 13 | 1.0 |
| Forehead | 50 | 184 | 8 | 0.6 |
| Chest | 29 | 196 | 9 | 0.3 |
| Volar side of forearm | 15 | 203 | 6 | 0.4 |
| Back of hand | 14 | 188 | 7 | 0.5 |
| a Data from Woodworth RS, Schlosberg H: Experimental Psychology. New York,
Holt, Rinehart and Winston, 1965.
b Arranged in descending touch-spot density. | ||||
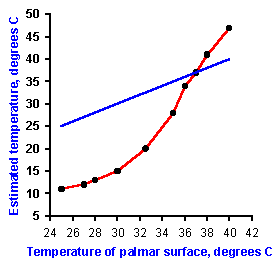 |
Fig. 5-7. Psychophysical intensity function for the perception of temperature of the palmar surface of the hand, as a function of the actual skin temperature after 30 min adaptation. Estimates of temperature (left ordinate) and relative intensity estimates (right ordinate) are plotted against actual temperature (abscissa) Average values for 18 subjects (Hensel H: Correlations of neural activity and thermal sensation in man. In Zotterman Y [ed]: Sensory Functions of the Skin in Primates, with Special Reference to Man. Oxford, Pergamon Press, 1976). |
In estimating skin temperature, people are quite accurate in the region of normal body temperature, 37 to 38 ºC, but they consistently overestimate higher and underestimate lower temperatures. This can be seen in the graph of Figure 5-7 of estimates of the temperature of the palm of the hand. The inaccuracy is extreme at low temperatures; people estimated the temperature to be 10 ºC when it was actually 25 ºC. When the temperature of the skin is changed rapidly, the sensation evoked depends not only on the amount and direction of change, but also upon the temperature from which it is changed, the acclimation temperature. This is illustrated in Figure 5-8 where acclimation temperature is plotted on the abscissa against the change in temperature on the ordinate. Starting at a temperature on the abscissa, to which the skin has been adapted for some time, the skin temperature had to be changed by the number of degrees on the ordinate to elicit a sensation of either warmth (reading upward) or cold (reading downward). Thus, starting at 28 ºC, the temperature has to be raised by about 1 ºC (reading upward from 0 to the heavy curve then across to the ordinate) to elicit a sensation of warmth or lowered by 0.15 ºC to elicit a sensation of cold (reading downward from 0 to the heavy curve then across to the ordinate). On the other hand, if the acclimation temperature is 38 ºC, the skin must only be warmed by about 0.1 ºC to elicit a sense of warmth, but it must be cooled by more than 0.6 ºC to elicit a sense of cold. To convince yourself that these observations are accurate, try the following experiment: Fill three bowls with water: one lukewarm, one cold and one warm. Put the left hand in cold water, the right in warm water for a while and then place both in the lukewarm water. A clear sensation of warmth will occur in the left hand and a sensation of cold in the right. An important conclusion from Figure 5-8 is that the same temperature can feel either warm or cold depending upon stimulus conditions, i.e., the acclimation temperature.
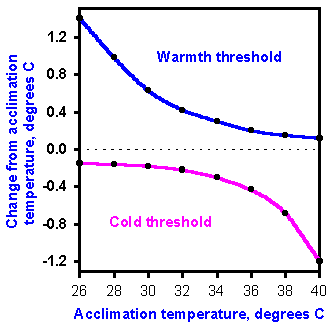 |
Fig. 5-8. The dependence of thresholds for sensations of warmth and cold upon the initial temperature of the skin. The skin was brought to one of the temperatures on the abscissa and held there for some time. Then the temperature was either raised or lowered until a sensation of warmth or cold was reported, and the threshold value was plotted on the ordinate as either an increase or decrease from the initial temperature expressed in degrees centigrade. Two curves were generated, one for warmth thresholds and one for cold thresholds. Values presented are accurate for temperature changes in excess of 6 degrees/min (Kenshalo DR: Correlations of temperature sensitivity in man and monkey: A first approximation. In Zotterman Y [ed]: Sensory Functions of the Skin of Primates, with Special Reference to Man. Oxford, Pergamon Press, 1976). |
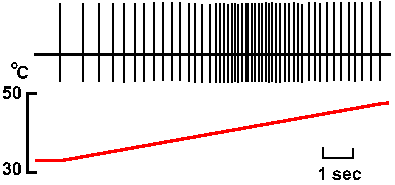 |
Fig. 5-9. The response of a single warm fiber to gradual warming of the skin in its receptive field. A monitor of the local temperature of the skin is shown in the lower trace whereas the spike discharge is shown in the upper trace (Redrawn from Hensel H: Plüger's Arch 313:150-152, 1969. |
If recordings are made from single primary afferent fibers, it is possible to identify warm fibers, which increase their frequency of discharge when the skin is warmed, and cold fibers, which increase their discharge frequency when the skin is cooled. The response of a single warm fiber is shown in Figure 5-9. Warm fibers are slowly adapting; they fire continuously at constant temperature. A monitor of the skin temperature at the bottom of the figure shows the temperature gradually increasing from 32 ºC to 50 ºC. As the temperature increases from 32 ºC to 44.5 ºC, the discharge frequency of the warm receptor increases, but further increases in temperature cause the discharge frequency to fall. This means that there is only one temperature that is uniquely signaled by the discharge frequency of the fiber, namely, 44.5 ºC, at which point the discharge frequency is maximum. Two temperatures--one above and one below 44.5 ºC--are signaled by every lower discharge frequency. Most people are able to detect changes in temperature as small as 0.08 ºC. The problem for the nervous system is to figure out what the skin temperature is by looking at the discharges of temperature receptors. One possible solution to the problem is to have different fibers, each with its own "best temperature," the temperature which yields the highest rate of discharge, but with best temperatures of the population of fibers distributed across the whole range of temperatures that humans sense. This would be a pattern or ensemble code. Unfortunately, the system does not seem to be built that way. Figure 5-10 shows the frequency responses of a number of warm fibers. Most of them have about the same "best temperature," namely 44.5 ºC. How they signal the changes in temperature to account for our ability to discriminate changes in temperature remains a mystery, but it seems likely that the manner of coding must be in terms of the pattern of activity across a number of fibers.
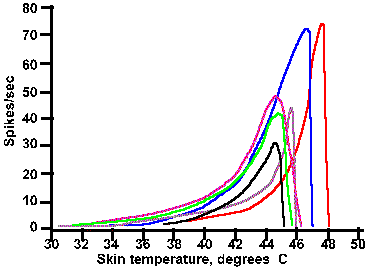 |
Fig. 5-10. Plots of the responses of six warm fibers. The frequency of discharge of each fiber (to a series of temperatures as in Fig. 5-9) is plotted against the temperature. Note that all curves reach a peak in the region from 44.5 ºC to 47.5 ºC (Hensel H and Kenshalo DR: J Physiol (Lond) 204:99-112, 1969). |
The threshold temperature for pain nociceptors (receptors that respond to damaging stimuli a human would find noxious) that are sensitive to noxious heating is about 44-46 ºC. That means they are beginning to discharge (presumably signaling pain) at the temperature at which the response rate of most warm receptors is beginning to fall off from its maximum (see Fig. 5-10). The temperature range from 38-44 ºC could be distinguished by the central nervous system from that above 44 ºC by sensing the discharge of thermal nociceptors that would be zero in the former case but not in the latter.
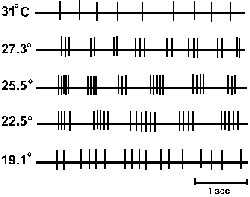 |
Fig. 5-11. The response of a single cold fiber to 5 different temperatures. At 31 ºC the fiber fires more or less continuously, fires in bursts in the range from 27.3 to 22.5 ºC, and then fires more or less continuously at lower temperatures (Iggo A and Iggo BJ: J Physiol (Paris) 63:287-290, 1971). |
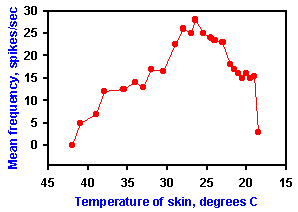
How cold sensations are coded by cold fibers may be easier to understand. Figure 5-11 shows the discharge of a single cold fiber at several temperatures. As the temperature is lowered from 31 ºC to 27.3 ºC, the discharge changes from a more-or-less continuous stream of impulses to a series of bursts. Also, as the temperature reaches 19.1 ºC, the fiber again returns to a more-or-less continuous discharge. If the average frequency of discharge is computed and plotted against temperature, the curve in Figure 5-12 upper results. It resembles the bell-shaped curve of warm fibers, yielding the same ambiguity for temperature determination. If, on the other hand, the number of impulses in a burst is plotted against temperature, as in Figure 5-12 lower, an approximately linear relationship results with no ambiguity over the range in which the bursts occur. However, this is only a part of the range over which temperature is sensed and discriminated by humans. lt may be that other such cold fibers respond in different parts of the temperature range so that the entire range is covered by the aggregate of cold fibers.
 |
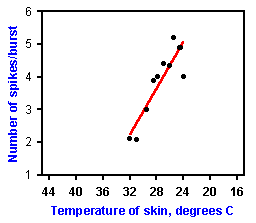 |
Fig. 5-12. Top: A plot of the mean frequency of discharge of the cell in Fig. 5-11 against skin temperature showing the usual increase in frequency followed by a decrease as the temperature decreases. Bottom: A plot of the number of impulses in the bursts evident in the range 27.3 to 22.5 ºC in Fig. 5-11 against the temperature in that range. Note the linear relationship between temperature and discharge frequency (Iggo A and Iggo BJ: J Physiol (Paris) 63:287-290, 1971). |
Prior experience with a stimulus can cause that stimulus to be perceived as either more or less painful, depending upon the nature of the experience. Painful stimulation, repeated in a psychological trauma-producing situation, may tend to make similar stimulation in the future more painful, whereas painful stimulation, repeated in otherwise pleasant surroundings, may tend to make future stimulation less painful.
Parental attitudes about pain and painful stimulation have a big influence on our responses to noxious stimuli. To some extent, these are determined by our culture; some cultures do not experience pain in certain situations where most others do. In some cultures, women say that they do not experience pain during childbirth; in some cultures, the husbands experience pain during childbirth. As judged from the absence of cardiovascular and respiratory changes that normally accompany pain, these women, in fact, do not experience pain, whereas their husbands do. Why not and why? There are also many examples of cultures in which people undergo, during religious ceremonies, treatments we would find excruciating, but they do not experience any pain, at least in the ceremonial context.
In some situations, especially emotional ones, a stimulus that would normally be perceived as painful does not evoke pain. For example, football players, during football games, and soldiers, during battles, can sustain serious injuries that, if they occurred in another situation, would be very painful, but in this situation are not accompanied by pain.
Similarly, a person anticipating a painful stimulus or anxious about his situation, will usually perceive a stimulus as more painful than he would if he did not anticipate the stimulus. For example, a person shown a hot iron, then blindfolded and led to believe he will be touched with the iron, may swear he has been burned when he has been touched with an icicle(5). Conversely, a painful stimulus may feel less painful if an innocuous stimulus is anticipated instead.
A person's sensitivity to pain also depends upon his immediately preceding sensory experiences. For example, people who live in cold climates are acutely aware that, after spending time outdoors without gloves on cold days, even lukewarm water can "burn" the skin painfully, though no damage is actually being done by the water.
Most people believe that pain is primarily a signal that body tissues are being or have been damaged, and it certainly does have qualities that are consistent with this notion. For example, in many cases, painful sensations are accompanied by a rapid withdrawal of an injured limb from the damaging stimulus. Persons with congenital pain insensitivity frequently injure themselves severely-bad burns, broken bones, appendicitis (unnoticed)-without being aware of it, because they have neither pain sensations nor changes in blood pressure, heart rate, or respiratory rate that accompany them. One of the enigmas of pain is that it can occur without injury and injury can occur without pain. We have already discussed some examples of injury without pain. Spontaneous pain, for example, phantom limb, causalgia, or neuralgia pain, can sometimes persist long after damaged tissues have healed and nerves have regenerated.
Most methods of measurement treat pain as if it were a single unique quality that varies only in intensity-mild versus moderate versus intense. However, pain is a complex category of experiences. Careful observations of pain sensations have shown that all pains are not the same. Pain from the skin is localized accurately, is variable in intensity and duration, but is invariant in quality or tone. No matter how pain is produced in skin, the sensation is always of the same quality, that is, if tests are done properly, the subject cannot say accurately how the pain was produced or whether receptors or nerve fibers were stimulated. Brief stimuli, such as hair pull, heating, pin prick, electrical shock, if applied without associated sensory (but painless) stimuli, all lead to a "pricking" sensation. The sensations only differ if the subject has some other clue, for example, non-noxious movement of nearby hairs, that tells him what the nature of the stimulus was. Prolonged stimuli of sufficient intensity all lead to "burning" sensations even if the stimulus is not a heat stimulus. Thus, pricking pain is a short-duration pain; burning pain is a cutaneous pain that continues.
| Pricking pain is a short-duration pain; burning pain is a cutaneous pain that continues. |
Muscle pain, pain from tendon, periosteum and joint, and pain from mucous membranes are all rather diffuse, difficult to locate precisely, continuous and distinct from cutaneous pain. Muscle pain is indescribable, but disagreeable and inconstant in intensity. Tendon, periosteal, and joint pain are similar in quality to muscle pain, but more constant in intensity. These and muscle pain are often referred to as "aching" pains. The mucous membrane-buccal membranes, conjunctiva, nasal membranes and glans penis-all are hypersensitive (relative to other tissues) to pain; slight contact or friction can lead to pain of surprising intensity. The quality of this pain also is similar to muscle pain.
Some observers have distinguished superficial (cutaneous) from deep (muscle, periosteal, tendon, joint, and mucous membrane) pains. Superficial pain stimuli lead to withdrawal of the limb (for a discussion of the withdrawal reflex, see Chapter 15) or kicking, if applied to the shoulder region in animals. Deep pain stimuli do not. In humans, superficial pain is associated with brisk movements, increased pulse rate, and a sense of invigoration, whereas deep pain is associated with quiescence, decreased pulse rate and blood pressure, sweating, and nausea. Deep pain is sometimes referred to as "sickening," a term never applied to superficial pain. Cutaneous nociceptors are activated by mechanical injury, chemical irritation, ischemia, thermal energy, and certain kinds of electrical stimulation. Deep pain is elicited by ischemia, prolonged muscle contraction, mechanical force, chemical irritation, distension, and spasm.
However, pain is not simply sensation. Some people have even suggested that pain is better classified as a need-state, like hunger, than as a sensation. Clearly, pain has a sensory component and can be induced by noxious stimuli impinging upon receptors. On the other hand, receptors are not required. The existence of phantom limb pain, painful sensations referred to an amputated limb, demonstrates this clearly. Peripheral nerves are not required either; central pain mechanisms are clearly able to generate pain after dorsal rhizotomy.
Pain also appears to have a memory component that can be triggered by stimulation or triggered spontaneously. In a fascinating experiment, teeth on both sides of a subject's mouth were drilled and filled without local anesthesia (the absence of sensation). As long as 70 days later, pin pricks of the nasal mucosa led to pain in the filled teeth. This pain was permanently eliminated on one side after recovery from a single injection of novocaine to block part of the trigeminal nerve on that side; but pain persisted on the unblocked side.
| Affective pain depends upon an intact connection to the prefrontal cortex; discriminative pain persists after lobotomy. |
All pain has two psychological aspects: one discriminative, that is, we can objectively gauge its intensity, location, and quality, and the other affective or emotional, pain causes suffering. It is important to distinguish the discriminative aspect from the affective aspect of pain. The importance of this distinction is highlighted by the fact that the two aspects can be dissociated by the proper clinical maneuvers, suggesting that different parts of the nervous system are involved. For example, separation of the prefrontal lobes from the rest of the cerebral cortex in the patient with intractable pain leaves the patient with his pain sensation intact, but the pain no longer bothers him. The suffering is eliminated even though the pain is not. The affective aspect of pain depends upon the integrity of cerebral cortical function; the discriminative aspect apparently does not.
If pain is so complex, can it really be defined at all? Clearly, from what we have discussed so far, the dictionary definition-a more or less localized sensation of discomfort, distress or agony, resulting from stimulation of specialized nerve endings; serving as a protective mechanism insofar as it induces the sufferer to remove or withdraw from the source-is not adequate for clinical pain; where a receptor may not be involved. Another definition-an abstract concept that refers to (1) a personal, private sensation of hurt; (2) a harmful stimulus that signals current or impending tissue damage; (3) a pattern of responses which operates to protect the organism from harm (Sternbach RA: Pain, A Psychophysiological Analysis, New York; Academic Press, 1968)-suggests a bit more of the individual variation, but still links pain inextricably to receptors. Dr. Ronald Melzack has suggested that pain is definable in terms of a multidimensional space that comprises subjective experiences that have both somatosensory and negative-affective components and elicit behavior aimed at stopping the conditions that produce them (Melzack R: The Puzzle of Pain, New York, Basic Books, 1968). This form of definition removes pain from its bond to receptors but still links pain to behaviors that may or may not be directly associated with the sensations. For example, the withdrawal reflex normally occurs with painful stimulation of the foot, but it begins before the pain sensation and, during general anesthesia, occurs in the absence of pain sensation (or in the absence of any pain the patient can later report). In Chapter 15, we shall see that the mechanism of the withdrawal reflex is localized to the spinal cord, higher centers are not required, but the mechanism of pain sensation requires supraspinal structures.
Pain is clearly not a simple phenomenon, and we must keep certain principles in mind in our discussion of it. These principles are as follows:
- There is not a one-to-one relationship between the intensity of noxious stimulation and the intensity of pain it produces.
- The same injury can produce different pain sensations and responses in different people as a result of cultural variables or prior experience.
- The same injury can produce different pain sensations and responses in the same person at different times, i.e., different times of day (pain sensitivity is lower in the morning than in the afternoon) or different times during the menstrual or other cycle.
- Psychological variables can intervene to produce variable pain sensations and responses.
- Pain perceived varies with the situation in which it occurs and the meaning of the situation for the individual.
- Pain varies with sensory adaptation levels.
- Pain refers to a category of complex experiences, not a single one.
- Clinical pain may not be the same or have the same neurological mechanism as laboratory or normal pain.
Because pain is so variable, it is not easy to study experimentally, yet some aspects of pain sensation have proven amenable to scientific investigation.
| The Neurophysiology of Pain |
| Activity in some small myelinated and unmyelinated fibers is associated with pain sensations. |
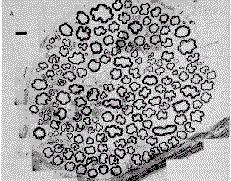
The fibers in a peripheral nerve are of different sizes-ranging from less than 1 m to 22 microns in diameter. A cross section of a nerve is shown in Figure 5-13 upper, and a distribution of fiber diameters is plotted in Figure 5-13 lower. The majority of fibers are less than 2 microns in diameter. These fibers are mostly unmyelinated fibers, termed C fibers. There is also a smaller group of fibers with diameters from 1-4 microns, termed A-delta fibers, and another group with diameters of 8-22 microns, termed A-alpha and A-beta fibers. The A-fiber groups consist of myelinated fibers. The threshold of a fiber to externally applied electrical shocks is inversely related to the diameter of that fiber. Accordingly, A-alpha fibers would have the lowest electrical threshold (require least current to evoke a discharge), A-delta fibers slightly higher, and C fibers the highest.
 |
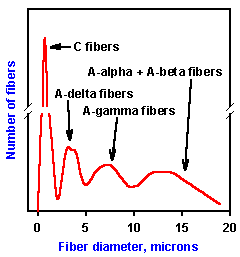 |
Fig. 5-13. Distribution of fibers within a peripheral nerve. Upper: A cross section of the posterior articular nerve of the cat showing fibers of different diameters. Calibration: 10 microns (Courtesy of F.J. Clark). Lower: A plot of the number of fibers of different diameters in a peripheral nerve. Note the predominance of fibers less than 2 microns in diameter. |
It was discovered during stimulation of cutaneous nerves in volunteer medical student subjects, that pain was not evoked by shocks to the nerves until the shocks were of sufficient strength to stimulate A-delta fibers. (This strength excites A-alpha and A-beta fibers but does not excite C fibers.) It was, therefore, concluded that A-alpha and A-beta fibers do not signal pain, but that at least some A-delta fibers do. Similarly, stimulation of C fibers is associated with unbearable pain. Because of the timing of the threefold pain sensation just described, it is likely that the first pain sensation is due to activity in A-delta fibers, and second and third pain waves are due to activity in C fibers.
An indicator that bright and dull pain are due to activity in fibers of different diameters and therefore different conduction velocities (see Chapter 12 for a discussion of the relationship between fiber diameter and conduction velocity) is the observation that two distinct sensations, bright and dull, are felt for painful stimulation on body parts far from the spinal cord-hands and feet-but not on parts close to the spinal cord-hips, back, and shoulders. The conduction distances from those parts nearer to the central nervous system are so short that the signals in larger (faster) and smaller (slower) fibers arrive nearly simultaneously, and the sensations therefore fuse into a single one. From farther away, the signals arrive at distinctly different times, and the sensations are separate.
When recordings are made from single C fibers during short application of noxious heat, an initial, high-frequency discharge is observed followed by a period of silence. After the period of silence the fibers resume their discharge but at a lower rate than the one observed initially. The rate of discharge in this latter phase is increased by warming the burned area of skin to a temperature that did not influence the cell previous to the burn. Thus, it seems that the transmission of pain information is accountable by activity in both A-delta and C fibers.
The study of nociceptors, receptors that respond only to stimuli that are strong enough to produce damage and are painful to humans, is a fairly recent development in neurophysiology because these fibers are small and easily damaged during dissection and, as we shall see later, contribute little to whole-nerve recordings, the compound action potentials. These receptors have interesting properties that may have important implications for pain research, but one should be cautious in applying the term "pain receptors" to them. This term implies that activity generated in these nociceptors leads predictably to pain. It is possible that some nociceptor activity leads to a withdrawal reflex or cardiovascular reflexes, but that activity does not lead to pain sensations. All that is known is that they can detect the existence of a noxious event or environment, that is, that they are nociceptors.
Some nociceptors respond only to painful mechanical stimuli and produce little or no discharge when the skin is damaged by extremes of heat or cold; nor do they respond to acid placed in cuts across the receptive field. The receptive fields of these nociceptors are made up of a number of spots of sensitivity separated by regions of insensitivity. The axons of these nociceptors are small fibers with diameters in the A-delta range. C fibers with similar properties are rarely found. Another group of A-delta fibers responds to damaging heat and damaging mechanical stimuli. Among C fibers there are some that respond to damaging stimuli, both mechanical and heat, and others that respond to both damaging mechanical and cold stimuli. The majority of C fiber nociceptors appear to be of this type, called polymodal nociceptors because they respond to more than one type of noxious stimulus.
Table 5-2
Pressure and Pain Thresholds on Various Parts of the Bodya | ||
Location |
Pressureb (g/mm2) |
Painc (g/mm2) |
| Tip of finger | 3 | 300 |
| Back of hand | 12 | 100 |
| Calf of leg | 16 | 30 |
| Abdomen | 26 | 15 |
| Back of forearm | 33 | 30 |
| a Data from Woodworth RS, Schlosberg,H: Experimental Psychology. New York, Holt
Rinehart and Winston, 1965.
b Tested with a human hair c Tested with a needle | ||
It is interesting that the topographic distributions of sensitivity to touch-pressure stimuli and pain stimuli are inverse. The thresholds for both pressure and pain are give in Table 5-2. In general, where sensitivity to pain is high, sensitivity to touch is low and vice versa. This phenomenon may reflect receptor density to some extent. The rough inverse relationship between touch spot and pain spot density shows clearly in Table 5-1.
| The Enteroceptors |
Originally, it was thought that the sense of the position of the joint, that is, the angle between the bones of the joint, was signaled by the myelinated fibers of the articular nerve leaving that joint. Recent studies indicate that most of these fibers do not, in fact, signal the static position of the limb. This is because they fail to discharge at any position but the extremes of flexion and extension. In addition, anesthetizing the human knee joint does not diminish position sense for that joint. It appears that the most likely candidate for signaling joint angle would be the muscle spindle receptors or group Ia or II afferent fibers (these will be discussed in detail in Chapter 11).
The receptors of the joint may be signaling that the joint is undergoing or about to undergo some undue stress or strain, thus serving a role in protection of the joint itself, not in body orientation. The majority of the fibers serving the joint are small-diameter myelinated and C fibers whose functions are unknown at present, but it is unlikely that they signal joint angle, because their very slow conduction velocities would cause long time delays in our ability to sense changes in joint angle. Such delays do not occur.
Other subcutaneous receptors
The Pacinian corpuscle is a rapidly adapting mechanoreceptor. Its distribution in the mesentery, joint capsule, connective tissues and at pressure points on the palmar and plantar surfaces of the extremities suggests that it may have a number of roles.In the carotid bodies, aortic bodies, and along the arteries and perhaps veins are receptors that play a role in the control of respiration. These receptors sense the amount of oxygen and CO2 in the blood and the pH of the blood, and trigger reflex changes in respiration volume and rate. In the carotid body, special receptor cells, innervated by the carotid branch of the glossopharyngeal nerve, increase their frequency of discharge in response to a decrease in blood pH of 0.1, a decrease in blood O2 saturation of 4%, and increases in blood CO2 concentration.
Near the carotid body is a structure called the carotid sinus. In the carotid sinus and also in the aortic arch are mechanoreceptors that sense blood pressure. These receptors increase their discharge rates with increases in blood pressure and decrease their discharge rates in response to decreases in blood pressure. The impulses from these receptors flow into the medullary region of the nervous system and modulate or initiate changes in heart rate and contractility, and in the diameter of blood vessels, that result in compensatory changes in blood pressure.
There are receptors in the heart that sense the volume of blood in the various chambers, and there are receptors in the lungs to signal the amount of inflation. Evidence exists that there are receptors in the hypothalamus that sense the temperature and osmolality of the blood and perhaps also the concentration of glucose in the blood. There are undoubtedly receptors in various parts of the body, including the brain, that can sense the circulating levels of hormones in the blood. A complete description of these kinds of receptors is beyond the scope of this discussion.
| Summary |
Somatic receptors are classed as either enteroceptors or exteroceptors depending upon whether they sense what happens inside or outside the body. Cutaneous exteroceptors come in a variety of anatomical forms which correlate roughly with different sensations. Free nerve endings are usually (but not always) associated with pain, temperature, and crude touch sensations, whereas encapsulated endings are usually (but not always) associated with light touch and pressure sensations. Mechanical sensations are signaled by both slowly and rapidly adapting receptors. The receptive field of a sensory nerve fiber is the area of skin over which a stimulus excites that nerve cell. The sizes of the receptive fields of primary afferent fibers are usually larger more proximally than distally. Physiologists distinguish two types of temperature sensitive fibers: warm fibers and cold fibers. Both probably signal temperature in the temporal patterns of their spike discharges or in ensemble codes. Pain sensations have discriminative and affective aspects. The affective aspects depend upon prefrontal cortical functions; discriminative aspects apparently do not. The first, bright (cutaneous) pain sensation is probably due to activity in A-delta fibers, whereas the second, dull pain sensation is probably due to activity in C fibers. Pain sensitivity is distributed approximately inversely to touch-pressure sensitivity-where pain sensitivity is relatively high (abdomen), touch-pressure sensitivity is relatively low.
| Suggested Reading |
- Burgess PR, Perl ER: Cutaneous mechanoreceptors and nociceptors. In Iggo A (ed): Handbuch Sensory Physiology II, pp. 25-32, Heidelberg, Springer, 1972.
- Burgess PR, Petit D, Warren RM: Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol 31: 833-848, 1968.
- Clark FJ, Burgess PR: Slowly adapting receptors in cat knee joint: Can they signal joint angle? J Neurophysiol 38:1448-1463, 1975.
- Iggo A, Young TW: Cutaneous thermoreceptors and thermal nociceptors. In Kornhuber HH (ed): The Somatosensory System, pp. 1-22, Acton, MA, Publishing Sciences Group, Inc., 1971.
- Melzack R: The Puzzle of Pain. New York, Basic Books, 1973.
- Sternbach RA: Pain. A Psychophysiological Analysis. New York, Academic Press, 1968.
- Wall PD: On the relation of injury to pain. Pain 6:253-264, 1979.
- Zotterman Y (ed): Sensory Function of Skin in Primates, with Special Reference to Man. Oxford, Pergamon Press, 1976.
Footnotes:
1. The idea of a sensation called crude touch as described here is found in most textbooks of neurology and neurophysiology, but if you search your experiences you will probably find nothing like crude touch. Perhaps there really is such a thing, or perhaps it's just what remains after there is a partial sensory loss.
2. This is an unfortunate terminology, but for the present we are stuck with it. Do not confuse type I and type II cutaneous primary afferent fibers with group I and group II afferent fibers from muscle and skin, to be discussed in Chapter 11.
3. The threshold stimulus, 1.6 m/msec, has been subtracted from abscissa values. To find actual stimulus velocity, add 1.6 to values on the abscissa.
4. A device similar to a compass, used by draftsmen to measure line segments in architectural drawings.
5. Of course, this depends a great deal on the acting skills of the experimenter.
[TOC] [Chapter 6] [Glossary] [Index] [Abbreviations]