 Chapter 12
Chapter 12 
PERIPHERAL NERVES
| The compound action potential |
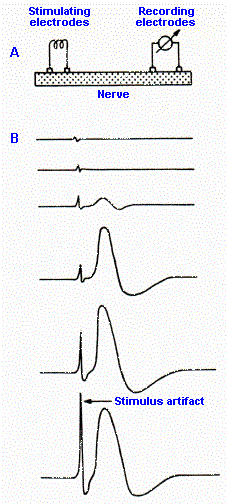 |
Fig. 12-1. A. Setup for recording the compound action potential from the sciatic nerve. B. Sample records taken at stimulus strengths increasing from top to bottom. Trace deflects upward when left recordng electrode is negative with respect to the right one. Note the graded nature of the compound action potential. (Katz B: Nerve, Muscle and Synapse. New York, McGraw-Hill, 1966) |
If we took a 10-mm length of mixed peripheral nerve, for example, the sciatic nerve, and recorded changes in electrical potential from one end of it while electrically stimulating the other end, as illustrated in Figure 12-1A, we could make some interesting observations that tell us a great deal about peripheral nerves. Sample records from such an experiment are shown in Figure 12-1B(1). In the uppermost trace, the strength of the stimulus is very low, and we see no response from the nerve. This stimulus strength is subthreshold. If the strength is raised, a tiny response appears in the record and, as the strength is increased even more, the response grows to a maximum value (next to bottom trace); further increases in shock strength do not further augment the response. The stimulus strength that just gives a response is termed a threshold stimulus; any stimulus of greater strength is suprathreshold. The strength that just gives the maximal response is a maximal stimulus; any strength greater is supramaximal. The response of the nerve is called the compound action potential. The compound action potential is graded in nature, in striking contrast to the all-or-none response of single axons.
There is a simple explanation for why the response of a nerve is graded and that of a single fiber is all-or-none. The nerve is composed of many fibers of different diameters, with these different diameters seemingly distributed at random throughout the nerve. It turns out that the threshold for discharge of an axon in response to externally applied current is inversely related to the diameter of the axon, so that for a large and small axon equidistant from the stimulating electrode, the large axon will have the lower threshold. Furthermore, if two axons of the same size are at different distances from the stimulating electrode (that is, one is buried deeper in the nerve or on the other side of the nerve from the electrode), the one closer to the electrode will pick up more current and therefore reach threshold at a lower stimulus strength than the one farther away. Thus, as the stimulus strength was increased in Figure 12-1B, the largest axons closest to the electrode began responding at lowest stimulus strengths. Then, at higher strengths, smaller axons close to the electrode and large axons farther away from the electrode responded, and their individual action potentials were added to the compound action potential. Finally, at maximal stimulus strength, every axon in the nerve was contributing its own all-or-none action potential, whence no further increase in the response was possible. It should be kept in mind that at all strengths, the recorded response is the sum over time of the action potentials of all of the discharging cells.
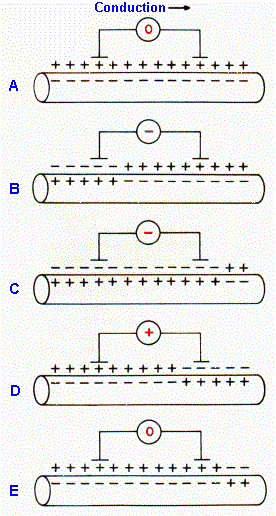 |
Fig. 12-2. Axon membrane polarization at various times during conduction of an action potential. Shown are polarizations when the spike is approaching the segment (A), under one electrode (B, D), under both electrodes (C), and retreating from the segment (E). The meter, connected to two external electrodes, shows the polarity of the recorded voltage. |
The shape of the compound action potential in Figure 12-1 may look like a temporally expanded action potential followed by an after-hyperpolarization; however, the positive potential of the compound action potential does not represent a membrane hyperpolarization. After-hyperpolarizations are really too small to contribute much to the compound action potential. Rather, the biphasic nature of the potential results from the bipolar extracellular recording setup. Note that two electrodes were used for recording, one closer to the stimulus, one farther away, and the voltage was recorded at one electrode with respect to the other, i.e., the difference in potential was recorded, but neither electrode was over inactive tissue. Figure 12-2 shows the events that occur as the compound action potential is propagating under the electrodes(2). In A, the action potentials have not yet reached the recording site; the membrane potential of most cells is at rest. (Note: Some cells discharge spontaneously.) Both electrodes are at the same potential; the difference is zero as shown. The advancing spikes have reached the first electrode in B; many cells have reversed their membrane polarities, and the potential at the first electrode has become negative with respect to the second. In C, the action potentials have begun to invade the membranes under the second electrode; the potential at the first electrode is still more negative than at the second but less so than in B. The membranes under the first electrode are already repolarized as the action potentials have passed, but they are still hypopolarized under the second electrode in D. The potential at the first electrode is now positive with respect to the second. In E, the spikes have passed both electrodes; the electrodes are at the same potential again, and the difference is again zero. The recorded configuration is therefore a result of the recording setup.
The compound action potential is an artifact of the manner in which the nerve was stimulated. It is
doubtful that such a potential is often elicited in nerves under normal conditions of sensation or
movement, but they may occur in some special circumstances. For example, in muscle nerves, when a
person jumps off an object and lands on the ground, a relatively synchronous volley of action potentials
is set up in the group Ia afferent fibers from the quadriceps muscles, and this in turn results in a relatively
synchronous volley in the alpha motoneurons of the quadriceps muscles, as we will see in Chapter 15.
Usually, however, there is a constant, asynchronous trickle of action potentials coming into the central
nervous system and also leaving it. However, the study of the compound action potential has given us a
lot of information about conduction in peripheral nerves.
| Types of fibers in peripheral nerves |
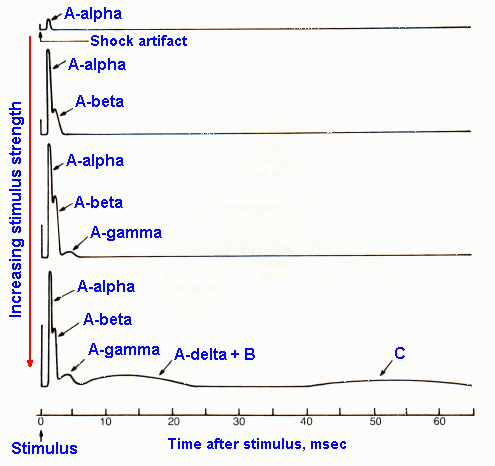 |
Fig. 12-3. Sample records from the sciatic nerve of responses elicited by electrical stimuli applied to the nerve 80 mm away. The various components of the compound action potential are labeled where they appear. Shock strengths increase from top to bottom. |
If, now, a greater length of sciatic nerve is used in the same experiment (say 80 mm of nerve), a new phenomenon appears in the recording. Figure 12-3 shows a stimulus-intensity series (increasing strength from top to bottom) recorded under these conditions. At just suprathreshold strength, a response (the A-alpha response) appears in the record at about 2 msec after the shock artifact. At higher shock strengths, this response grows in size, and a new response (the A-beta response) appears in the record about 4 msec after the artifact. Further increases in shock strength result in further increases in both the alpha and beta responses and the addition successively of the new responses: A-gamma, A-delta and B, and C. Based on the discussion of the precious paragraph, it is appropriate to assume that the components added to the response at higher stimulus strengths represent the responses of successively smaller fibers, and this is indeed the case. Notice also that the responses of successively smaller fibers occur later and later in time after the artifact. This is because the conduction velocity of an axon is a direct function of its diameter, and it requires a longer time for the action potentials in more slowly conducting axons (smaller ones) to travel the 80 mm than for action potentials in more rapidly conducting axons (larger ones).
These different responses weren't seen in the experiment in Figure 12-1 because the short conduction distance in that experiment caused the action potentials in all sizes of fibers to arrive at the recording electrode at nearly the same time; there wasn't sufficient time for differences in conduction velocity to show up. The individual responses were all bunched up together and were thus superimposed in the record. Nevertheless, the same fibers were discharging at the same shock strengths in both experiments.
Take a moment at this point to note in Figure 5-13, that the smallest fibers are the most numerous by far, whereas the largest are least numerous. In cutaneous nerves, measurements have indicated that there are about twice as many A-delta fibers as A-alpha fibers and about 4.6 times as many C fibers as A fibers(3). Though this is the case, the maximal response (Fig. 12-3) recorded from the largest fibers, the A-alpha fibers, is many times larger than that recorded from the smallest fibers, the C fibers. This is because fibers contribute voltage to the recording in proportion to the square of their diameters. The result is that a fiber 2 times as large as another contributes 4 times as much voltage to the recording. In our case, the largest A fiber is 22 micrometers in diameter, the largest C fiber only 1 micrometer in diameter; they differ by a factor of 22, and they contribute voltage to the recording in the ratio of (22)2:(1)2 or 484:1.
Two Systems for Classifying Axons in Peripheral Nerve by Diameter
| Type of fiber | Diameter, micrometers | Conduction velocity, m/sec | General Function |
| A-alpha | 13-22 | 70-120 | alpha-motoneurons, muscle spindle primary endings, Golgi tendon organs, touch |
| A-beta | 8-13 | 40-70 | touch, kinesthesia, muscle spindle secondary endings |
| A-gamma | 4-8 | 15-40 | touch, pressure, gamma-motoneurons |
| A-delta | 1-4 | 5-15 | pain, crude touch, pressure, temperature |
| B | 1-3 | 3-14 | preganglionic autonomic |
| C | 0.1-1 | 0.2-2 | pain, touch, pressure, temperature, postganglionic autonomic |
Roman Numeral System
| Type of fiber | Diameter, micrometers | Conduction velocity, m/sec | General Function |
| Ia | 12-20 | 70-120 | muscle spindle primary endings |
| Ib | 11-19 | 66-114 | Golgi tendon organs |
| II | 5-12 | 20-50 | touch, kinesthesia, muscle spindle secondary endings |
| III | 1-5 | 4-20 | pain, crude touch, pressure, temperature |
| IV | 0.1-2 | 0.2-3 | pain, touch, pressure, temperature |
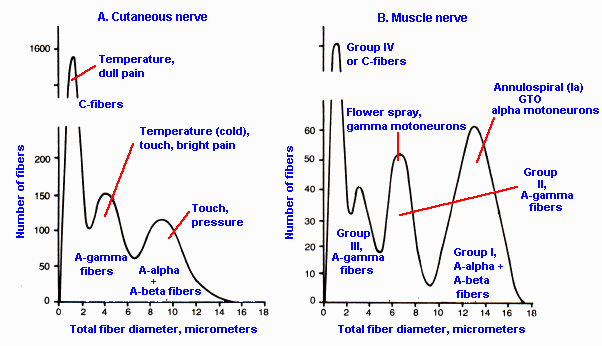 |
Fig. 12-4. Fiber diameter spectra for a cutaneous (A) and a muscle nerve (B) to indicate the types of fibers in each and the use of the naming systems of Table 12-1. (Boyd IA, Davey MR: Composition of Peripheral Nerve. Ediburgh, Livingstone, 1968) |
We find that fibers of particular receptor systems are often separable to some extent on the basis of their conduction velocities. Table 12-1 summarizes the two different classification schemes for peripheral neurons based upon their conduction velocity or diameter. The Roman numeral scheme (lower part of the table) refers to afferent fibers only, whereas the letter scheme (upper part of the table) includes both afferent and efferent fibers. It may help to note that A-alpha, Ia and Ib fibers are mostly of muscle origin or destination, and C and group IV fibers are all unmyelinated. Normally, we use the letter classification scheme to designate cutaneous-afferent fiber types and the Roman numeral scheme to designate muscle-afferent fiber types, but unfortunately this is not always the case, so care must be exercised. The fiber diameter spectra of a muscle nerve (B) and a cutaneous nerve (A) are indicated in Figure 12-4 for comparison with classifications in Table 12-1. The sum of the two fiber spectra in Figure 12-4 is approximately equivalent in shape to that in Figure 5-13. Be careful not to confuse the fiber spectra of Figure 12-4 with the compound action potential of Figure 12-3.
| Properties of different types of nerve fibers |
Nerve fiber types differ in their response to local anesthetics, in their susceptibility to anoxia, and in their susceptibility to pressure block. Using the letter classification, these differences are summarized in Table 12-2. B fibers are most influenced by anoxia, A fibers are most susceptible to pressure block, and C fibers are most readily blocked by local anesthetics. You may be acutely aware that hypoxia blocks A fibers before C fibers if you are in the habit of sleeping with your arm under your head. Upon waking, you may commonly observe that your sense of touch is dulled (you may have a tingling sensation), but your pain sensitivity is either normal or perhaps even slightly greater than normal.
Table 12-2
Susceptibility of Different Types of Fibers to
Conduction Block by Various Agents
| Effect | Most susceptible | Intermediate | Least susceptible |
| Block by hypoxia | B | A | C |
| Block by pressure | A | B | C |
| Block by local anesthetics | C | B | A |
| The measurement of conduction velocities |
It is common in peripheral nerve damage that the conduction velocity is slowed drastically in the damaged portion. For example, in carpal tunnel syndrome, where the bones of the wrist are broken in such a way that the fragments put pressure on the median nerve, partially blocking conduction, it is possible to assess the extent of the damage by measuring the conduction velocity of the nerve and comparing it with that in the normal arm.
Measurement of the velocity is straightforward. First, two sets of stimulating electrodes are placed on the skin over the median nerve, one near the top of the forearm and one 10 cm closer to the wrist. It is important that this distance is measured accurately or, at least, that it is the same on both arms if they are to be compared. Next, a pair of surface electrodes or needle electrodes, pushed through the skin into the belly of the abductor policis brevis muscle, is used to record the muscle activity. Recordings are made from muscle because recordings from nerves are more difficult to obtain. The position of the stimulating electrodes can be checked by stimulating and looking for abduction and flexion of the thumb, the action of the abductor policis brevis. Electrode position is adjusted to obtain the lowest threshold for contraction with both pairs of stimulating electrodes. The nerve is then stimulated with each set of electrodes, while looking at the electromyogram, EMG, from the muscle and conduction time between the shock artifact and the beginning of the EMG response is measured. Ten centimeters divided by the difference between the conduction times for the two electrodes is the maximum conduction velocity for axons in the nerve. Two pairs of electrodes are used instead of just one because this procedure eliminates the need to estimate and compensate for the utilization time in the nerve, i.e., the time it takes for the membrane potential to reach the critical firing level and discharge a spike, and the delay in transmission at the neuromuscular junction, that is, the time it takes for chemical transmission (see chapter on synapses). In the normal individual the velocity should be nearly the same in both arms.
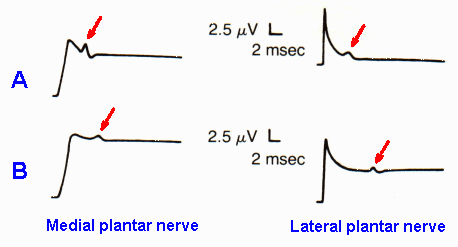 |
Fig. 13-5. Recordings of compound action potentials (arrows) in the medial and lateral plantar nerves in a normal person (A) and in a patient with tarsal tunnel syndrome (B). Note that tarsal tunnel syndrome is accompanied by slowing of conduction and reduction in amplitude of the compound action potential. (Oh SJ, Sarala PK, Elmore RS: Ann Neurol 5:327-330, 1979) |
Tarsal tunnel syndrome is the lower limb equivalent of carpal tunnel syndrome, but rarer. In tarsal syndrome, there is compression neuropathy of the posterior tibial nerve associated with burning pain and paresthesia in the toes and on the sole of the foot. Figure 12-5 shows recordings from the medial and lateral plantar nerves of a normal person (A) and a patient with tarsal tunnel syndrome (B). Note that the compound action potentials are smaller and later in the patient than in the normal person, because of the slowing of conduction in some fibers and block of conduction in others. Conduction velocities measured in normal records are 41.25 and 34.80 m/sec respectively, whereas those in the patient are 26.10 and 17.78 m/sec, a slowing of 37% and 49%, respectively.
| Summary |
The compound action potential is the graded response of a peripheral nerve to electrical stimulation. It is graded because axons of the nerve are of differing diameters, and their thresholds to externally applied current vary with diameter. The conduction velocity of an axon is a function of its diameter, so the fastest component of the compound action potential is contributed by the fastest and largest fibers, whereas later components are contributed by slower and smaller fibers. Fibers contribute voltage to the compound potential in proportion to the square of their diameters. The result is that large fibers, though they are fewer in nerves, give the largest components to the compound action potential. There are two classification systems for peripheral nerve based on conduction velocity. The letter scheme is for both afferent and efferent fibers. A and B fibers are myelinated; C fibers are not. The Roman numeral system is only for afferent fibers. Groups I, II and III are myelinated, group IV is not. The letter scheme is most often used for cutaneous fibers, the Roman numeral scheme for muscle afferent fibers. B fibers are most sensitive to hypoxic block; A fibers are most sensitive to pressure block; and C fibers are most susceptible to anesthetic block.
| Suggested Reading |
- Hodgkin AL: Evidence for electrical transmission in nerve. Part I. J Physiol (Lond) 90:183-210, 1937.
- Hodgkin AL: Evidence for electrical transmission in nerve. Part II. J Physiol (Lond)90:211-232, 1937.
- Junge D: Nerve and Muscle Excitation. Sunderland MA, Sinauer, 1976.
- Katz B: Nerve, Muscle, and Synapse. New York, McGraw-Hill, 1966.
- Lloyd DPC: Neuron patterns controlling transmission of ipsilateral hind limb reflexes in cat. J Neurophysiol 6:293-315, 1943.
- Patton HD: Special properties of nerve trunks and tracts. In Ruch TC, Patton HD (eds): Physiology and Biophysics, 19th ed. Philadelphia, WB Saunders, 1965.
Footnotes:
1. 2. 3.
[TOC] [Chapter 13] [Glossary] [Index] [Abbreviations]