 Chapter 11
Chapter 11
MUSCLE RECEPTORS
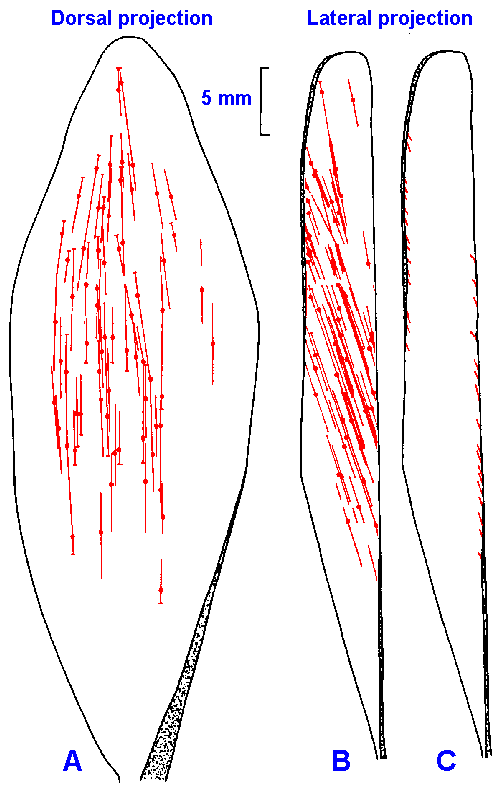 |
Fig. 11-1. The distribution of muscle receptors in the medial gastrocnemius muscle of the cat. The locations and orientations of spindles are shown in dorsal (A) and midsagittal (B) views. The locations of Golgi tendon organs are shown in a midsagittal view only (C). The aponeuroses of the muscle are indicated by shaded areas. (Swett JE, Eldred E: Anat Rec 137:453-460, 1960) |
So far in our discussion of receptors we have dealt only with exteroceptors. Now we will deal briefly with three kinds of enteroceptors, all found in muscle. These are sometimes termed proprioceptors, because they sense what goes on in the body itself(1). Primary and secondary muscle spindle receptors and Golgi tendon organs all send information about the state of the muscle to the central nervous system. All muscles, with the exception of extraocular and facial musculature, contain all three types of receptors. The spindle receptors sense muscle length and the rate of change of muscle length, whereas the Golgi tendon organ senses muscle tension and the rate of change of muscle tension. We have already seen that receptors in the joints do not signal the angle of the joint. It is likely that muscle spindle receptor messages provide the information the central nervous system uses to compute the angle of joints. In addition, all types of receptors in muscle provide information used in systems that control movement and posture.
| Muscle spindle afferent fibers |
The primary and secondary
muscle spindle afferent fibers both
arise from a specialized structure
within the muscle, the muscle spindle,
a fusiform structure 4-7 mm long and
80-200 m in diameter. The spindles
are located deep within the muscle
mass, scattered widely through the
muscle body, and attached to the
tendon, the endomysium or the
perimysium, so as to be in parallel with
the extrafusal or regular muscle
fibers. Although spindles are
scattered widely in muscles, they are not found throughout. Figure 11-1 shows the distribution of
spindles in the medial gastrocnemius of the cat, in dorsal (A), and midsagittal projections (B), and
for comparison the location of Golgi tendon organs (C). The aponeuroses of the muscle are
indicated by shaded areas. It can be seen that nearly 40% of the muscle is devoid of spindles,
especially the marginal areas. A drawing of a muscle spindle is shown in Figure 11-2. It contains two
types of intrafusal muscle fibers (intrafusal = inside the fusiform spindle): the nuclear bag
fibers and the nuclear chain fibers. The nuclear bag fibers are thicker and longer than the
nuclear chain fibers, and they receive their name from the accumulation of their nuclei in the
expanded bag-like equatorial region-the nuclear bag. The nuclear chain fibers have no equatorial
bulge; rather their nuclei are lined up in the equatorial region-the nuclear chain. This distinction is
illustrated in Figure 11-3. A typical spindle contains two nuclear bag fibers and 4-5 nuclear chain
fibers.
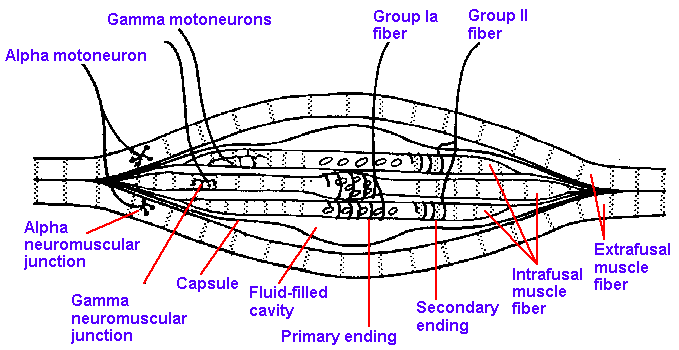 |
Fig. 11-2. Drawing of a muscle spndle to show the nature of attachment, the arrangement of the intrafusal fibers, and how the afferent and efferent fibers enter the spindle. |
The sensory innervation of the muscle spindle arises from both group Ia and group II afferent fibers. As shown in Figure 11-3, a single, large group Ia fiber coils around the equatorial regions of both nuclear bag and nuclear chain fibers, forming the annulospiral endings or primary muscle spindle receptors. There appears to be only one group Ia afferent fiber per spindle, but every intrafusal muscle fiber within that spindle receives innervation from that fiber. Current thought is that all group Ia afferent fibers form annulospiral endings, and therefore the terms primary muscle spindle afferent fiber and group Ia afferent fiber are used interchangeably. The smaller group II fibers terminate at either end of the nuclear region primarily on the nuclear chain fibers (there is apparently some innervation of bag fibers by secondary muscle spindle afferent fibers, but there is disagreement as to how much); they form flower-spray endings or secondary muscle spindle receptors. There usually are several group II fibers innervating each spindle. Not all group II fibers form such endings, so these terms are not synonymous.
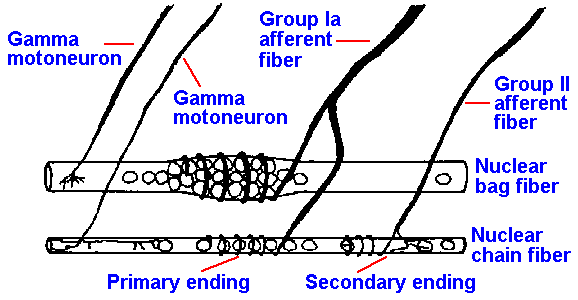 |
Fig. 11-3. A nuclear bag and a nuclear chain fiber showing their innervation by group Ia and Group II afferent fibers and gamma motoneurons. (Matthews PBC: Physiol Rev 44:219-288, 1964) |
The intrafusal fibers are striated muscle fibers that receive innervation from the fusimotor neurons or gamma-motoneurons. Activity in fusimotor neurons produces a contraction of the striated, polar regions of the bag and chain fibers, putting stretch on the equatorial region (where the receptor regions are) that has few myofibrils and therefore, has little contractility. It is apparently the stretching of this central region, regardless of how it is accomplished, that is the adequate stimulus for both primary and secondary spindle receptors. The fusimotor neurons or gamma-motoneurons should not be confused with the larger skeletomotor neurons or alpha-motoneurons, whose activity produces contraction of the extrafusal fibers that do the work of the muscle. The difference in diameter of fusimotor (gamma-motoneurons) and skeletomotor fibers (alpha-motoneurons) is illustrated in Figure 11-4. The former average about 5 m in diameter, the latter 13 m. Contraction of all the intrafusal fibers at once does not produce any measurable tension in the muscle. The intrafusal fibers are much shorter than the extrafusal fibers, 4-7 millimeters compared with 3-50 centimeters. Extrafusal muscle fibers can shorten by as much as 40% of their resting length, which for a 20-cm muscle would be 8 cm. The largest intrafusal fiber, shortening by the same percentage, would only change length by 2.8 mm. The extrafusal fiber is therefore capable of a 30-fold greater change in length. The average human striated muscle fiber has a diameter of about 60 micrometers; the intrafusal fiber averages about 10 micrometers. Because the force produced by a skeletal muscle is proportional to its cross-sectional area, the extrafusal fiber is capable of producing at least 36 times more force than the intrafusal fiber. Add these factors to the relative numbers of intrafusal (in cat soleus muscle about 300) and extrafusal fibers (again in cat soleus muscle about 25,000), and it is not hard to see why intrafusal fibers do not generate much force.
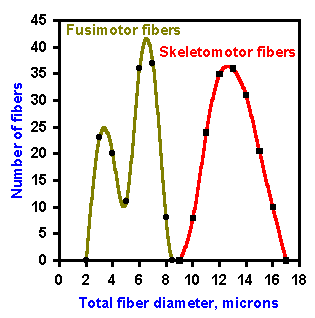 |
Fig. 11-4. Fiber spectrum of efferent portion of a muscle nerve. Indicated are A alpha and A gamma fibers, fusimotor and skeletomotor fibers. (Boyd IA, Davey MR: Composition of Peripheral Nerves. Edinburgh, Livingstone, 1968) |
It also appears that the mechanism for generating force in intrafusal muscle fibers may be different than in extrafusal fibers. There are no action potentials in intrafusal fibers as there are in extrafusal fibers (see Chapter 14), with a consequence that one striated end of an intrafusal fiber may contract without the other end doing so. This cannot happen in normal extrafusal fibers.
Stretching the equatorial region of the muscle spindle, the adequate stimulus for the receptor, may be accomplished by gamma-motoneuron activation and intrafusal muscle contraction. Another way to stretch the equatorial region of the spindle is to stretch the muscle (Fig. 11-5) and thereby stretch the entire spindle, because it is attached in parallel with extrafusal muscle fibers. Muscle spindle receptors respond to stretch of the muscle and signal muscle length and rate of change of length to the central nervous system. Both primary and secondary spindle afferent fibers give static or length-sensitive responses to stretch, i.e., they respond to maintained stretch in a sustained (tonic) fashion at a discharge frequency proportional to the length of the muscle (Fig. 11-6). Both primary and secondary muscle spindle afferent fibers usually discharge tonically when the muscle is at its resting length. When the muscle is stretched and held at some new length (left side of figure, lengthening is an upward deflection of stimulus trace), both types increase their discharge rates and maintain a discharge for as long as the new muscle length is maintained (an example of a slowly adapting response).
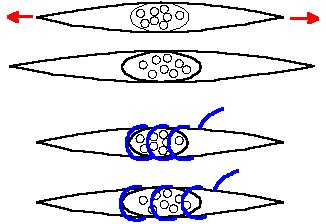 |
Fig. 11-5. Drawing depicting the stretch of the nuclear region of the spindle caused by stretching the muscle (upper pair) and by stimulation of gamma motoneurons, causing the striated intrafusal fibers to contract (lower pair). |
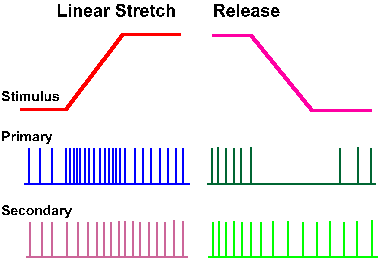 |
Fig. 11-6. Responses of spindle afferent fibers to muscle stretch. A monitor of the stretch (lengthening, upward deflection) is shown in the upper trace. The response of a primary spindle afferent fiber is shown in the second trace, that of a secondary spindle afferent fiber in the third. (Matthews PBC: Physiol Rev 44:219-288, 1964) |
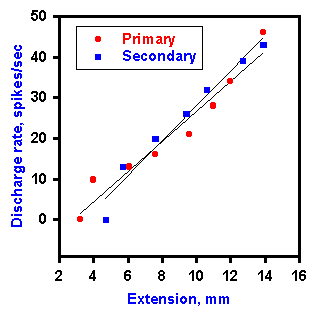 |
Fig. 11-7. A plot of the frequency of static discharge of a primary and secondary muscle spindle against the length of the muscle. Recordings were made with ventral roots cut. Primary resposes are plotted with filled circles. (Jansen JKS, Matthews PBC: Acta Physiol Scand 55:376-386, 1962) |
Only the primary muscle spindle afferent fiber gives a dynamic or velocity-sensitive response to muscle stretch. As the muscle length is being changed, the primary ending signals the rate at which it is being changed. The faster the muscle is stretched, the higher is the rate of discharge of the ending. Figure 11-8 shows the responses of both primary and secondary spindle endings to two stretches of the muscle at different rates. A monitor of the muscle length is shown in the upper traces, the response of the primary ending is shown in the second traces, and the response of the secondary ending is shown in the third traces of both A and B. Notice that the primary ending responds with a higher frequency during the faster stretch in B, even though the initial and final lengths are the same in each case. Also note that the rate of discharge decreases from its peak after the final length has been reached. This decrease defines the dynamic index, which is the difference between the dynamic response frequency for that rate of stretch and the static response frequency for that final length and serves as an indicator of rate sensitivity. When the velocity of stretch is zero, that is, when the muscle length is constant (phases 1 and 3 of Fig. 11-8), the discharge of the cell will be signaling only the length of the muscle. The primary spindle codes zero velocity with zero "velocity" discharge, but with an appropriate "length" discharge. When the muscle is changing length, that is, when the velocity is not zero, there will be a velocity response, as well as a length response that is appropriate for the length of the muscle at each given instant of time. Very near the end of phase 2 in Figure 11-8, the muscle has nearly reached its final length as in phase 3, but it is still changing length. Because its length is nearly that in phase 3, they can be taken as equal for a first approximation. This gives the following conditions:
|
Thus, the dynamic index can be used as an indicator of the velocity response. Figure 11-9 is a plot of the dynamic index versus velocity (or rate) of stretch for both primary and secondary endings. The curve for secondary endings is flat compared with that for primary endings, indicating that this receptor has little sensitivity to the velocity of stretch.
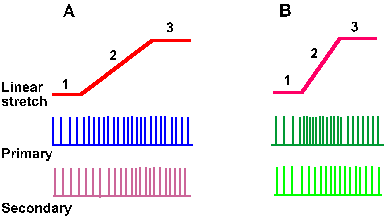 |
Fig. 11-8. The dynamic response. The monitors of the stretch at two different rates (A and B) are shown in the upper traces. Note that both stretches start and end at the same muscle length. The responses of a primary ending are shown in the second traces; those of a secondary ending are shown in the third traces. Note the higher frequency of discharge of the primary ending at the higher rate of stretch (B). Note that the length of the muscle is changing in phase 2 but is constant in phases 1 and 3 of these records. |
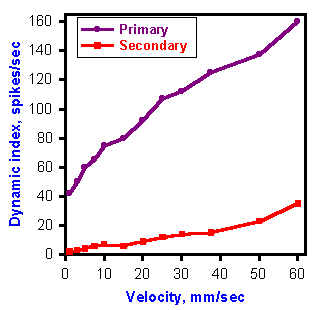 |
Fig. 11-9. A plot of the dynamic index versus the rate of stretch for a primary and a secondary muscle spindle afferent fiber. Note the flatness of the secondary curve. (Matthews PBC: J Physiol (Lond) 168:660-678, 1963) |
It is the dynamic response capacity of the primary muscle spindle ending that provides for the vigorous response to tapping the muscle tendon as the physician does in reflex testing (Fig. 11-10). The tap rapidly stretches the spindles (upper trace) and the primary endings respond to this rapid rate of stretching (second trace). The secondary endings, because they lack dynamic sensitivity, respond little if at all to the tap, which produces only a small change in the length of the muscle (third trace). After a brief stretch, the spindle returns to its original length, and the discharge of the primary ending stops and then it returns to its prestretch rate of discharge. This behavior will be important in our consideration of the tendon tap reflex(3)(Chapter 15).
Fusimotor effects on spindle afferent fiber discharges
Fusimotor neurons are physiologically separable into two groups based on their effects on the dynamic and static responses of the spindle afferent fibers. Activity in static fusimotor fibers increases the static responses of both primary and secondary spindle afferent fibers, whereas activity in dynamic fusimotor fibers increases the dynamic response in primary spindle receptors. These effects are illustrated in Figure 11-11. Trace A is again the monitor of muscle length and trace B is the response of a single primary spindle afferent fiber to the stretch. The effect is similar to that illustrated in Figure 11-5. During trace C, a single static fusimotor fiber was stimulated at 70/sec. The effect on the static stimulation was so marked that the cell increased its discharge at the initial length; it discharged more vigorously at its final length (but still signaled both lengths in its discharge); and the dynamic response was masked by the increase in the static response, resulting in a decrease in the dynamic index, the indicator of the velocity response. The same procedure, this time while stimulating a dynamic fusimotor fiber, produced a very marked change in the dynamic discharge, but had much less effect on the static response, although there was some increase (Fig. 11-11D).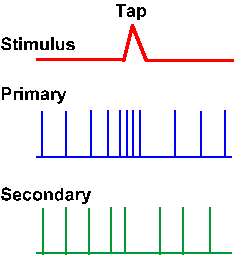 |
Fig. 11-10. Responses of primary and secondary muscle spindle afferent fibers to tapping the tendon of the muscle that they innervate. Monitor of change in length caused by tap is shown in upper trace; upward is an increase. (Matthews PBC: Physiol Rev 44:219-288, 1964) |
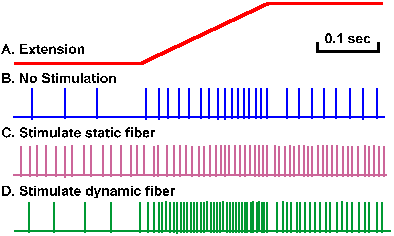 |
Fig. 11-11. Effect of gamma motoneuron discharges on spindle sensitivity. A. Monitor of the muscle length during a stretch. B. Response of a primary spindle ending to stretch. C. Response of the ending to the stretch during continuous stimulation of a single static fusimotor neuron at 70/sec. D. Response of the ending to the stretch during continuous stimulation of a single dynamic fusimotor neuron at 70/sec. (Crowe A, Matthews PBC: J Physiol (Lond) 174:109-131, 1964) |
Such changes in the discharge properties show up more clearly perhaps when the muscle is stretched and relaxed in a sinusoidal fashion rather than a linear fashion. Records made of the discharge of a primary muscle spindle afferent fiber are shown in Figure 11-12. The first and fourth traces show the action potentials discharged by the fiber. The second and fifth traces are a monitor of the stretch (upward deflection) and relaxation (downward deflection) of the muscle at 1 mm peak-to-peak at 3 Hz. This sinusoidal stimulus was applied to the muscle so that the zero position was approximately at the resting length, the muscle being stretched and relaxed equally around it. The two remaining traces show the period during the record when a dynamic fusimotor neuron (third trace) and a static fusimotor neuron (sixth trace) were stimulated (i.e., stimulation is on when the line is present, off when it is absent). At the beginning of each record there is a period of time when there was no fusimotor stimulation. The group Ia fiber discharges during the stretch of the muscle and ceases its discharge when the muscle is relaxed. The average length of the muscle was not sufficiently great in this experiment to provoke a continuous discharge from the fiber. The effect of dynamic fusimotor activity was to increase the rate of discharge during the stretch portion of the stimulus, but it did not induce the cell to discharge during the relaxation phase of the sine wave stimulus. The static fusimotor activity, on the other hand, converted the bursting response into a continuous response that occurred at a frequency appropriate for the average length of the muscle during the stimulus. A similar effect could have been achieved by increasing the length of the muscle before the sinusoidal stimulus was applied, i.e., by setting the zero position of the sinusoid at some length longer than the resting length.
The effects of fusimotor activity on spindle receptors are summarized in Table 11-1. From the distribution of the primary and secondary spindle afferent fibers and the nature of the effects it is possible to deduce that dynamic fusimotor fibers innervate primarily nuclear bag fibers, whereas static fusimotor neurons innervate nuclear chain or nuclear bag fibers or both. In a study of single chain and bag fibers, it was found that stimulation of a single dynamic gamma-motoneuron caused contraction of only bag fibers; chain fibers were not affected. Static gamma-motoneurons caused contraction of bag fibers only, chain fibers only, or both depending upon the static gamma-motoneuron.
Table 11-1
Comparison of Actions of Static and Dynamic Fusimotor Neurons
Phenomenon observed |
Dynamic neuron |
Static neuron |
| Static response of primary ending | Increase | Slightly larger increase |
| Static response of secondary ending | No effect | Increase |
| Dynamic response of primary ending | Increase | Decrease in dynamic index, though rate may increase |
| Dynamic response of secondary ending | No effect | Remains small |
| Frequency of occurrence | One-fourth of -fibers | Three-fourths of -fibers |
| Intrafusal fiber type affected | Nuclear bag | Nuclear chain or bag or both |
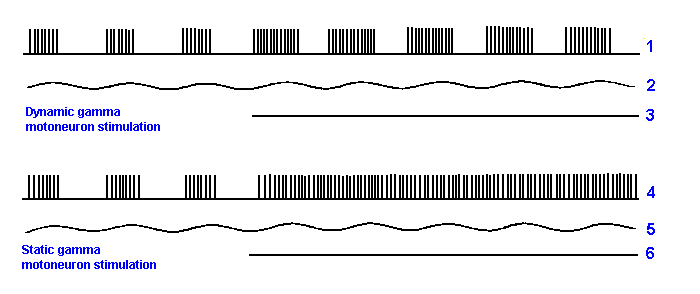 |
Fig. 11-12. The effect of fusimotor stimulation on the respnsiveness of the mammalian primary ending to a sinusoidal stretching of medium extent (1 mm peak-to-peak movement at 3 Hz). (Crowe A, Matthews PBC: J Physiol (Lond) 174:132-151, 1964) |
The important physiological effect of the fusimotor innervation of the spindles seems to be to alter the bias of the spindle afferent endings. Stimulation of a static fusimotor neuron increases the length discharge of both primary and secondary muscle spindle afferent fibers for any muscle length. This is shown in Figure 11-13A. The discharge rate without the fusimotor activity is plotted against muscle length as the lower line, as in Figure 11-7. With fusimotor activity a new relationship is generated parallel to, but slightly above the lower line. Actually, a family of such parallel lines would be generated by different rates of discharge in the gamma motoneuron. For any muscle length, the rate of discharge is greater with gamma activity than without, and it is greater by a constant amount.
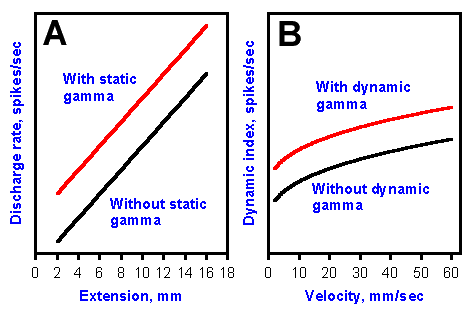 |
Fig. 11-13. Effects of fusimotor neuron activity on the sensitivity of the primary or secondary muscle spindle afferent fibers to length of the muscle (A) and of primary muscle spindle afferent fibers to rate of change of length of the muscle (B). |
Likewise, dynamic fusimotor neuron stimulation increases the velocity discharge of a primary muscle spindle afferent fiber for any velocity of stretch. Figure 11-13B shows two parallel curves relating velocity of stretch to the velocity discharge rate, expressed as the dynamic index. The upper curve is the discharge of the spindle under the influence of a gamma-motoneuron discharge; the lower is without such influence. Again, there would be a family of such parallel curves for different rates of gamma-motoneuron discharge. The velocity discharge rate is greater for any velocity with gamma-motoneuron stimulation, and it is higher by a constant amount. The effect of both static and dynamic fusimotor neurons on the muscle spindle receptors is to increase their discharge, in the one case, their discharge for the length of the muscle (static fusimotor neurons on both primary and secondary spindle afferent fibers) and in the other case, their discharge for rates of change of the length of the muscle (dynamic fusimotor neurons on the primary spindle afferent fiber). Many people are tempted to say that the gamma-motoneuron discharge increases the sensitivity of the spindle receptors, but actually the change is in a parameter called bias. A change in sensitivity would actually result in a change in the slope of the discharge rate-extension relation. The slopes of the relations with and without gamma-motoneuron activity are the same, the curves are parallel. The kind of change actually seen, i.e., that in Fig. 11-13A, is called a change in bias. Similarly, that seen in Fig. 11-13B is a change in bias.
It appears that there is ongoing fusimotor activity (gamma tone or gamma bias). Figure 11-14 shows the response of a primary spindle receptor to stretch (monitor in lower trace) when the ventral roots of the spinal cord are intact (second trace) and when they have been cut (upper trace). Recall that the gamma-motoneurons are efferent fibers, and they exit the spinal cord through ventral roots. The discharge of the primary spindle ending, both dynamic and static, is reduced by cutting the ventral root, suggesting that this fusimotor modulation is an ongoing thing, and that both dynamic and static fusimotor fibers are active in the absence of movement or stimulation. Position-discharges (the slope of the lines in Fig. 11-13A) can fall from as high as 10 impulses/sec/mm to four impulses/sec/mm when the ventral roots are cut. In addition, spindle receptors become silent at minimum lengths (see Fig. 11-7), a condition never obtained with ventral roots intact. Except during rapid shortening, primary endings discharge some spikes even at shortest muscle lengths in the presence of gamma bias.
It is reasonable to ask why sensory receptors like the muscle spindle receptors would have something like gamma bias. One likely reason can be seen by referring back to Fig. 11-13A. In the absence of gamma-motoneuron activity, the muscle spindle receptor would have a discharge of zero at a 1-mm length (extrapolate the lower curve back to the x-axis). In that condition, it would not be signaling anything to the brain about muscle length. But the job of the spindle receptors is to continuously signal muscle length. The brain can make the receptor signal something even at these shorter lengths if it makes the gamma-motoneurons active. In the presence of gamma-motoneuron activity, the receptor would have a non-zero discharge (read upward to the upper curve at 1 mm). So, what the gamma activity does is to force the spindle receptors to work in the center of their operating ranges, not at the ends. But, you might ask, isn't the information that the brain is getting about length false information? In a way, it is. But remember that it was the CNS that sent out the gamma signal. It knows how large that signal was, and presumably it knows what effect that would have on the spindle receptors. It might be just a matter of subtracting a length corresponding to the gamma signal.
| Golgi tendon organs |
The third muscle receptor of concern to us is the Golgi tendon organ. This receptor lies near the muscle-tendon junction (Fig. 11-15) or buried deep within the tendon itself. The receptor consists of specializations of the terminals of the group Ib afferent fiber itself, with a delicate capsule that surrounds the nerve which, in turn, surrounds several fascicles of tendon. The nerve fibers lie between fascicles in such a way that they can be "pinched" between them as the force is increased. This is how this receptor is thought to be activated. Group Ib seems to be composed entirely of Golgi tendon organ afferent fibers, so these terms are used interchangeably.
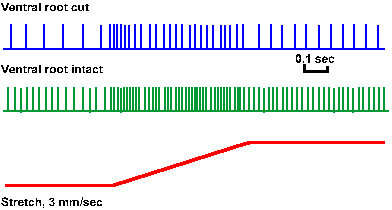 |
Fig. 11-14. Response of a primary spindle ending to stretching the muscle. Lower trace is amonitor of the muscle length. Middle trace shows the response to the same stretch with ventral roots intact; upper trace, with ventral roots cut. (Jansen JKS, Matthews PBC: J Physiol (Lond) 161:357-378, 1962) |
The Golgi tendon organ signals to the central nervous system the tension developed by the muscle during contraction or exerted on it during a stretch. For many years, it was thought that these receptors had high thresholds to muscle tension and participated in controlling muscle activity only at extremes of tension, functioning as a protective device. Actually, the tendon organs are relatively insensitive to tension applied to the muscle by stretching it, but they are extremely sensitive to tension developed by the muscle when it contracts. The reason for this is that the tension on tendon organs is different under passive and active conditions. A muscle like the cat's soleus muscle has many extrafusal muscle fibers in it (about 25,000), but only a few Golgi tendon organs (about 45). Each tendon organ is arranged in series with a few muscle fibers (average 7-10). When the muscle is stretched, the tension applied is distributed across all 25,000 muscle fibers, but the seven fibers associated with each tendon organ only experience 7/25,000 to 10/25,000 of it-quite a small amount. Also, the material of the tendon in which the Golgi tendon organs are located is stiffer at rest than the muscle is. When the resting muscle is stretched, much of the tension applied is used in stretching the relatively compliant muscle fibers; the stiffer tendon is not much affected. In addition, the tension caused by stretching the muscle is exerted on the tendons at an angle to the Golgi tendon organs, so that only the component of the force parallel to the muscle fibers actually stretches the tendon organ. This can be small, especially in pennate muscles. On the other hand, if the muscle contracts, the tension developed by the extrafusal fibers is transferred directly to the tendon organ associated with them. Not every tendon is innervated by a Golgi tendon organ. It appears that the system of tendon organs "samples" the average tension in various parts of the muscle in the same way spindle afferent fibers sample its length.
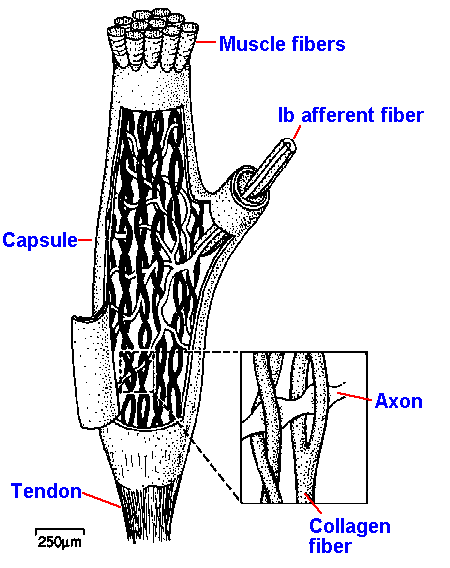 |
Fig. 11-15. The location of the Golgi tendon organ at the muscle-tendon junction. |
The response to muscle stretch (monitor in lower trace) of a Golgi tendon organ (second trace) is compared with that of a primary spindle ending (upper trace) in Figure 11-16. The tendon organ has both a dynamic and a static response, but it, of course, is signaling tension and rate of change of tension, rather than the length or rate of change of length that the primary muscle spindle signals. Because of the series elastic properties of the muscle, the tendon organ does not begin to discharge until long (in neurophysiological time) after the spindle ending has begun to discharge. The real distinction between the response of the two receptors is seen when an isometric twitch contraction is elicited in the muscle (Fig. 11-16). When the extrafusal fibers shorten (monitor in upper trace), the spindle is unloaded because it is attached in parallel, and the equatorial region relaxes. The result is that the primary spindle ending stops discharging (middle trace). On the other hand, when the muscle contracts isometrically, it develops tension, and the in-series-attached Golgi tendon organ increases its discharge (lower trace).
The differences between the three types of
muscle receptors are summarized in Table 11-2.
Although differences in location and sensitivity to
different forms of stimulation exist, these
receptors work together in controlling the activity
of the muscles with which they are associated, as
we shall see in another chapter.
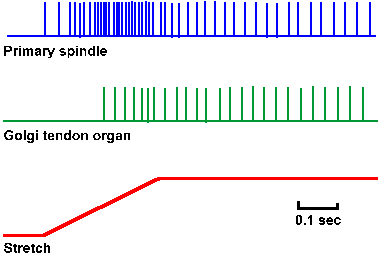 |
Fig. 11-16. Comparison of the responses of a primary spindle ending and a tendon organ. A monitor of the muscle stretch is shown in the lower trace and the response of the spindle ending in the upper trace. The response of the tendon organ is shown in the middle trace. Note the later start in the discharge of the tendon organ and the less vigorous response. (Matthews BHC: J Physiol (Lond) 78:1-53, 1933) |
Anatomical evidence suggests that among
muscle fascicles that contain muscle spindles,
Golgi tendon organs are usually present at the
muscle-tendon junction. This is shown in Figure
11-1B and C. This relationship suggests an even
closer cooperation than was previously expected.
Table 11-2
Comparison of Properties of Receptors in Muscle
Property |
Primary Ending |
Secondary Ending |
Golgi Tendon Organ |
| 1. Location | Mid-equatorial region of bag and chain fibers in spindles | Juxta-equatorial region of chain fibers in spindles | Muscle-tendon junction |
| 2. Afferent fiber | Large, group Ia | Small, group II | Large, group Ib |
| 3. Efferent control | Both static and dynamic fusimotor | Static fusimotor | None known |
| 4. Response to ramp stretch with plateau | Dynamic and static (signals length) | Static (signals length) | Dynamic and static (signals tension) |
| 5. Response to release of stretch | Abrupt silence | Progressive decrease | Abrupt silence |
| 6. Response to tendon tap | Low threshold, vigorous | High threshold, little | High threshold, vigorous if threshold is exceeded |
| 7. Sensitivity to small stretches | High, especially if rapid | Low | Low |
| 8. Response to twitch contractions | Abrupt silence | Abrupt silence | Vigorous discharge |
| 9. Signals | Muscle length and rate of change of length | Muscle length | Muscle tension and rate of change of tension |
| Summary |
Most mammalian striated muscles are supplied with three types of receptors that signal the contractile state of the muscle. The first is the annulospiral ending or the primary muscle spindle ending that innervates the equatorial region of the intrafusal muscle fibers of the muscle spindles. The second is the flower-spray ending or secondary muscle spindle ending that innervates the ends of the nuclear regions of primarily the nuclear chain, intrafusal muscle fibers. Both primary and secondary muscle spindle receptors signal the length of the muscle in a linear fashion as a frequency code. Primary spindle receptors also signal the rate of change of muscle length. Primary spindle endings are terminals of group Ia afferent fibers, whereas secondary endings are terminals of fibers in the group II range. The bias of both types of spindle receptors is controlled by the fusimotor neurons or gamma-motoneurons that innervate the muscular portions of the intrafusal muscle fibers and cause them to contract. Dynamic fusimotor neurons affect mainly the dynamic or rate responses of primary endings, whereas static fusimotor neurons affect mainly the static or length responses of primary and secondary spindle endings. Fusimotor neurons discharge tonically resulting in gamma bias or gamma tone. The third receptor in muscle is the Golgi tendon organ that innervates the tendon near the muscle-tendon junction and signals muscle tension and rate of change of muscle tension.
As a self-test, try filling in the following table. Use only I for increase discharge, D for decrease discharge, or N for no change.
Table 11-3
Change in Discharge Rates of Muscle Receptors
During Various Activities
Activity |
Primary Spindle |
Secondary Spindle |
Golgi Tendon Organ |
| Passive stretch | |||
| Isometric1 contraction | |||
| Isotonic1 contraction | |||
| Lengthening2 contraction |
1 Details of isometric and isotonic contractions can be found in Chapter 14.
2 A lengthening contraction occurs when the muscle contracts but is forced to lengthen by a load
it cannot lift.
Want to see the answer? Click Here
| Suggested Reading |
- Barker D [ed]: Symposium on Muscle Receptors. Hong Kong, Hong Kong Univ. Press, 1962.
- Bessou P, Pags B: Cinematographic analysis of contractile events produced in intrafusal muscle fibres by stimulation of static and dynamic fusimotor axons. J Physiol (Lond) 252:397-427, 1975.
- Bourne GH [ed]: The Structure and Function of Muscle, 2nd ed. Vol. I-III. New York, Academic Press, 1972.
- Matthews PBC: Muscle spindles and their motor control. Physiol Rev 44:219-288, 1964.
- Matthews PBC: Mammalian Muscle Receptors and Their Central Actions. Baltimore, Williams and Wilkins, 1972.
- Olkowski Z, Moncha SL: Muscle spindle. In Bourne GH [ed]: The Structure and Function of Muscle, 2nd ed, Vol. II, Part 2. New York, Academic Press, 1973.
Footnotes:
1. The term proprioceptor is usually reserved for receptors in muscle, tendon and joint, but it is sometimes used more loosely as a synonym for enteroceptor.
2. Recordings were made with ventral roots cut.
3. This is sometimes called the tendon jerk reflex.
[TOC] [Chapter 12] [Glossary] [Index] [Abbreviations]