GUSTATORY AND OLFACTORY SENSES
| Taste |
It is appropriate that we consider taste and smell together because they are so intertwined in our experience that most people are unaware that most of what they call taste is really an olfactory experience. The sensations evoked by a substance put into the mouth are complex and involve much more than taste. For example, a mouthful of orange soda pop gives one a taste that is a combination of sour and sweet; it is cold; it may sting or tingle a bit if it is carbonated; it evokes a complex touch sensation in the mouth, and it smells fruity or fragrant. The only part of this complex of sensations that is taste in origin, i.e., resulting from receptors on the tongue, palate or pharynx is the sensation of sweetness or sourness. Anyone who has ever had a head cold can attest to the flatness or blandness of his diet during that time. This is due to the fact that access of odorants to olfactory receptors is blocked by the copious secretions of mucus.
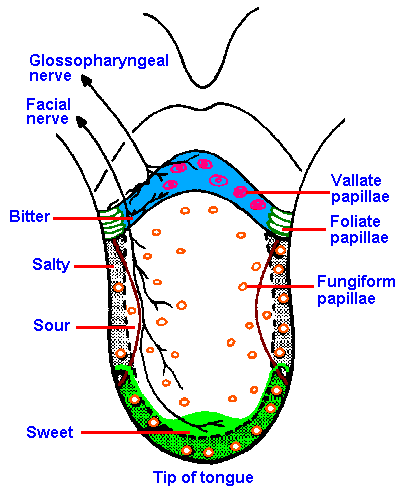 |
Fig. 10-1. The distribution of gustatory papillae, their innervation, and the regions of maximum sensitivity to different submodalities of taste on the human tongue. (Altner H: Physiology of taste. In Schmidt RF [ed]: Fundamentals of Sensory Physiology. New York, Springer-Verlag, 1978) |
The gustatory system is much simpler than the olfactory system. Four primary taste submodalities are generally recognized: sweet, sour, salty, and bitter. Different regions on the tongue exhibit different maximal sensitivities to the four taste submodalities (Figure 10-1 which also shows the pattern of innervation of the tongue). The tip of the tongue is the most sensitive to sweetness and saltiness. The sensation of sourness is experienced best on the lateral aspects of the tongue, and bitterness is experienced best and perhaps only on the back of the tongue. Next time you put some bitter substance such as tonic water (quinine) into your mouth, you can verify this for yourself.
The chemical senses are difficult to study experimentally because the stimuli that lead to gustatory sensations are not well understood. Sour tastes are evoked by all acids in dilute solutions. Apparently, it is the hydrogen ion that activates taste receptors and leads to a sensation of sourness. Accounting for salty tastes is more difficult. Sodium chloride is the only substance known to evoke a purely salty taste in any concentration that is suprathreshold. With other compounds, having one of the cations, Na+, K+, Li+, Ca2+, and one of the anions, Cl-, Br-, I-, SO42-, NO32-, CO22-, the saltiness varies with concentration. For example, a very dilute solution of KCl tastes sweet and, as the concentration increases, it becomes first bitter, then both bitter and salty, and finally purely salty. Both the anion and the cation are apparently involved in evoking the salty sensation.
The basis for sweet and bitter tastes is not known. Sucrose is a carbohydrate, a disaccharide formed from one molecule each of fructose (a fruit sugar) and glucose. Glucose, fructose, and starch are also carbohydrates. Fructose (grape sugar) is the sweetest; glucose is less sweet than sucrose or fructose; and starch is not sweet at all. Some alcohols are very sweet. For example, xylitol is used as a sweetener in Europe and in some chewing gum in the United States. Saccharin is also sweet (and bitter), but chemically it bears little similarity to either the sugars or the alcohols. The only known commonality for all these substances is that they excite gustatory receptors leading to sensations of sweetness.
The sensation of bitterness is evoked by many vegetable alkaloids, such as quinine, and by some metallic salts. Some people think that our sensitivity to bitterness may be a protective mechanism because many plant poisons are alkaloids and are bitter. However, the sense of bitterness may not be a real deterrent to ingesting bitter substances. Many of the favored foods of gorillas are found to taste bitter to humans. Either gorillas do not taste them as bitter or they are attracted to bitter substances. Interestingly, the substance phenylthiocarbamide tastes bitter to some people, but for others it is nearly tasteless, indicating that different people may have taste receptors with different response characteristics or that they may have different numbers of the same taste receptors.
There are also interactions between taste stimuli or sensations. Sucrose tastes sweeter after caffeine than after water; tartaric acid is more sour after KCl than after water; and caffeine is more bitter after sucrose than after water. After eating miracle fruit (a tropical fruit), even substances that are normally very sour taste sweet. You experience this sort of interaction any morning you eat grapefruit (it tastes very sour) after drinking a glass of sweet pineapple juice.
Taste sensitivity varies widely depending upon the submodality and the substance being tasted. For example, the absolute threshold for detection of sucrose (sweet) is a concentration of 0.02 M, whereas the threshold for sodium chloride is 0.035 M, for hydrochloric acid (sour) is 0.002 M, and for quinine sulfate (bitter) is 0.0000004 M. These absolute thresholds cover five orders of magnitude of concentration difference. Another substance that is sweet, saccharin, has a threshold of 0.00002 M, three orders of magnitude lower than that for sucrose. Taste thresholds for different submodalities are also influenced differentially by changes in temperature. Thus, the sensitivity to NaCl and quinine sulfate decrease with increasing temperature in the range from 17 to 42 °C; sensitivity to HCl is unaffected over the same range; and sensitivity to sweet substances increases. With the exception of sweet substances, none of the submodalities behaves as if it involved a chemical reaction, the rates of which are usually speeded by increasing temperature.
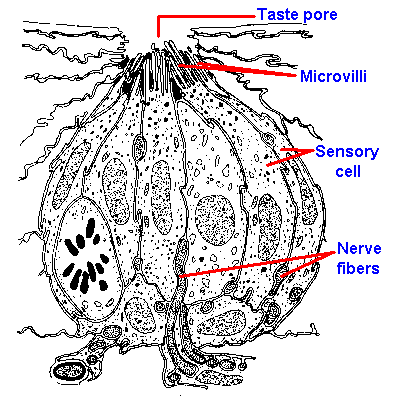 |
Fig. 10-2. Drawing of a cross section through a taste bud from a foliate papilla of a rabbit indicating the arrangement of receptor cells and the locations of the taste pore, microvilli, and nerve fibers. (Andres KH: Discussion of paper by Murray RG, Murray A: The anatomy and ultrastructure of taste endings. In Welstenholme GEW, Knight J [eds]: Taste and Smell in Vertebrates. London, Churchill, 1970) |
Taste anatomy
Gustatory receptor cells are found in the taste buds of fungiform, foliate, and circumvallate papillae located mainly on the tongue. The distribution of the different papillae on the tongue is shown in Figure 10-1. In the intact papilla, about all that can be seen from the surface, even with a scanning electron microscope, is a small hole, the taste pore, through which the sapid substance must pass. Inside is the taste bud, which is composed of elongated sensory cells arranged with other nonsensory cells like the segments of an orange, as shown in Figure 10-2, which is a drawing of a cross-section through a taste bud. The apical portions of the sensory cells have microvilli that project into the region of the taste pore. These microvilli are 2 to 5 µm long and 0.05 to 0.2 µm wide, and function perhaps to increase the surface area of the cell membrane. There are 40 to 60 densely packed and interdigitated taste cells per bud. It is unlikely that substances can get between the densely packed cells down to the sensory nerve fibers, so it must be the cells themselves that respond to chemicals. Diffusion of sapid solutions in the direction of the nerve fibers is also impeded by a substance found between the sensory cells at their apical ends. The location of this substance is shown by the blackened areas in Figure 10-2. Even if a taste substance could get between the cells, it could not get to the nerve fibers that are buried, sometimes completely, in invaginations of the sensory cells. For these reasons, it is unlikely that sapid substances excite the sensory nerves directly, but more likely indirectly through the sensory cells.It is known that taste sensory cells are amongst the shortest-lived cells in the body. It has been estimated that taste cells live only 250+/-50 hours. The cells are formed from undifferentiated epithelial cells, and their formation is presumably under neural control. (In fact, the taste buds depend upon the intact nerve for existence; cut the nerve they degenerate, allow the nerve to grow back and they reappear.) The taste sensory cells begin on the periphery of the bud and move toward the center in a matter of a few days; then they die. This means there is a constant, rapid turnover in taste cells, implying that the specific sensitivity of the sensory cell is determined by the nerve fibers that innervate it. If it were otherwise, our sense of taste would be constantly changing, whereas taste is relatively stable over time.
Beneath the taste bud there is a plexus of myelinated nerve fibers of 1- to 6-µm diameter that enter the bud. The smaller diameter fibers associate with only one sensory cell, but the larger fibers associate with two or more cells. A given sensory cell may receive contacts from up to 30 nerve fibers. In addition, a given nerve fiber can innervate up to nine separate taste papillae.
Afferent fibers carry impulses from the tongue to the brain stem in the chorda tympani branch of the seventh cranial nerve, the lingual branch of the ninth nerve, and the pharyngeal branches of the tenth nerve. The chorda tympani innervates the anterior 2/3 of the tongue, whereas the lingual innervates the posterior 1/3. The vagus innervates mainly the pharyngeal and laryngeal areas (see Fig. 10-1). In addition to taste signals, these nerves also carry temperature and tactile information from their receptive fields. Taste fibers in these nerves tend to be A-delta fibers with conduction velocities of 2-18 m/sec. There may be pathways for taste fibers other than the three nerves previously mentioned.
In the brain stem, taste nerves relay in the nucleus tractus solitarius where they contact secondary cells that are organized in a roughly topographical manner. These secondary cells then project fibers to the medial part of the ventral thalamus near the somatic face projection from the trigeminal nerve and also to a pontine taste area. The cortical projection sites appear to be in the pre- and postcentral gyri near the face representation and in the insular region.
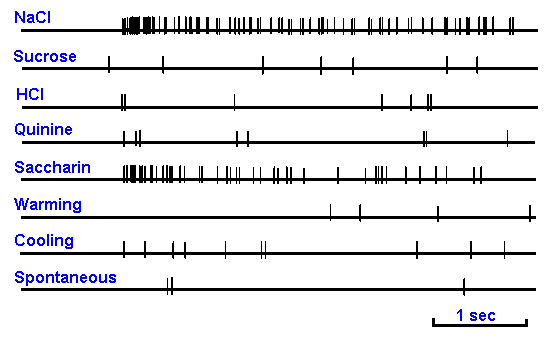 |
Figure 10-3. Impulse discharges in a single chorda tympani nerve fiber of a rat. Responses elicited by application to the tongue of 0.1 M NaCl, 0.5 M sucrose, 0.01 N HCl, 0.02 M quinine hydrochloride, 0.02 M sodium saccharin, 40°C water and 20°C water. Spontaneous discharges are shown in the bottom trace. Between traces the tongue was rinsed with 25°C water. (Ogawa H, Sato M, Yamashita S: J. Physiol (Lond) 199:223-240, 1968) |
Taste physiology
Taste neurons normally respond to several different kinds of chemicals so that chorda tympani taste fibers typically respond to substances that are salty, bitter, sweet, and sour. That is, they appear to respond to stimuli of two or three or even four different taste submodalities. An example of the discharges evoked in a single taste fiber in the chorda tympani nerve by substances flowing over the tongue is shown in Figure 10-3. This particular nerve cell gives a brisk discharge when NaCl and saccharin flow over the tongue, but it is only minimally, if at all, excited by sucrose, HCl, or quinine. It is also not especially sensitive to changes in the temperature of the fluid bathing the tongue. The question then is: How can we distinguish sweet from sour when our taste receptors respond nonspecifically to taste stimuli?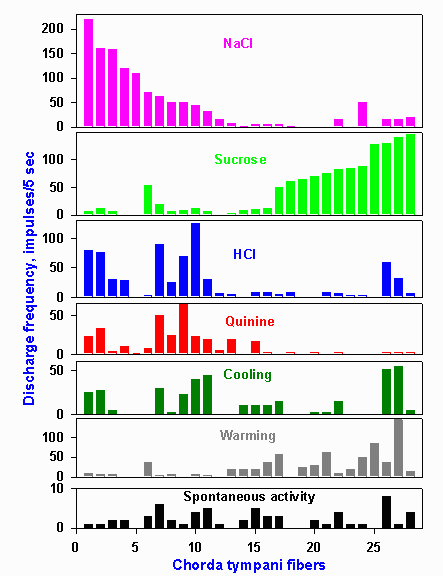 |
Figure 10-4. Response profiles of 28 hamster chorda tympani fibers. Stimuli were 0.1 M NaCl, 0.5 M sucrose, 0.01 N HCl, 0.02 M quinine hydrochloride, 20 C water and 40 C water. The response of a single fiber to each stimulus is found by reading up columns indicated by the letters on the abscissa. (OgawaH, Sato M, Yamashita J: J Physiol (Lond) 199:223-240, 1968) |
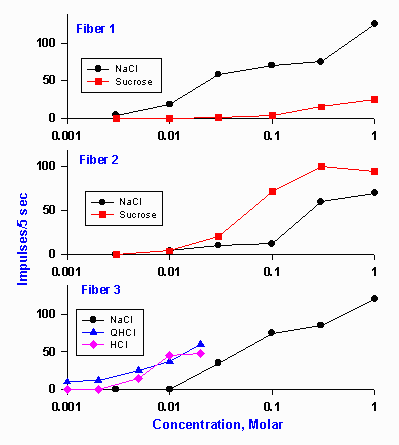 |
Figure 10-5. Concentration-response magnitude relationships in three typical chorda tympani fibers of rats. Ordinate indicates the number of impulses discharged by the fiber in the first 5 sec after the substance was applied. Fiber #1 was predominantly sensitive to NaCl, fiber #2 was more sensitive to sucrose than NaCl, and fiber #3 was sensitive to NaCl, quinine, and HCl. (O Ogawa H, Sato M, Yamashita J: J Physiol (Lond) 199:223-240, 1968) |
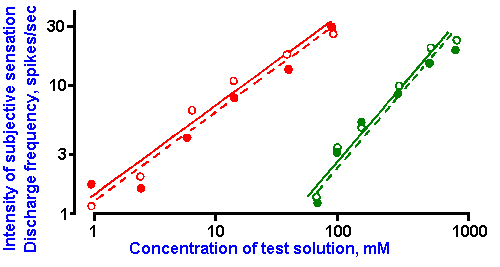 |
Figure 10-6. Dependence of subjective intensity of taste sensations (open circles) and of the frequency of discharge in fibers of the chorda tympani nerve (filled circles) upon the concentration of citric acid (red) and sucrose (green) solutions. Log-log plot. The slopes of the lines correspond to the exponents, k, of power functions with k=0.85 and 1.1. (Borg G, Diamant H, Strom L et al: J Physiol (Lond) 192:13-20, 1967) |
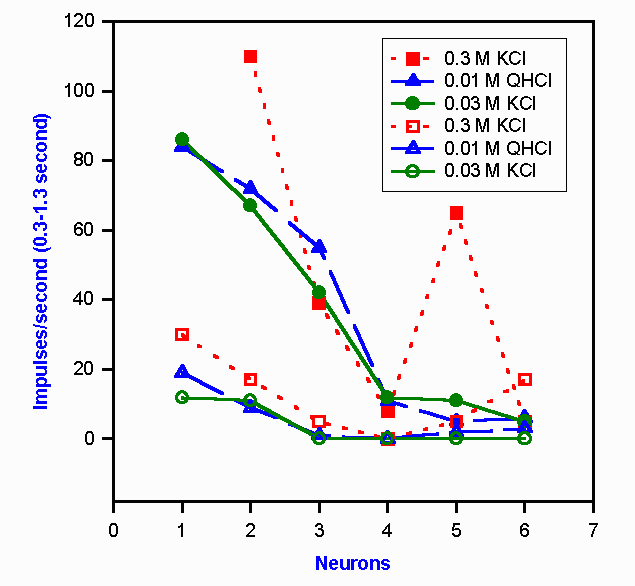 |
Figure 10-7. Neural representation of a change in taste quality with a change in the concentration of KCl. Six chorda tympani neurons are indicated by the numerals on the abscissa. The responses of these chorda tympani fibers (open symbols) and nucleus tractus solitarius neurons (filled symbols) to two different concentrations of KCl and one of quinine hydrochloride are shown by the six sets of curves. To a human, 0.3 M KCl is salty, 0.2 M KCl is bitter, and 0.01 M QHCl is bitter. (Doetsch GS, Ganchow JJ, Nelson LM et al: Information processing in the taste system of the rat. In Pfaffmann C [ed]: Olfaction and Taste, III. New York, Rockefeller Univ Press, 1969) |
However, the problem of intensity coding is not always a simple one. It should be recalled that there is an interaction between quality and intensity (concentration) for some substances, e.g., KCl. For those substances, the response of the cell ensemble to a given concentration should be like the response to another substance that yields the same sensation. That this does occur is shown in Figure 10-7. In this graph are plotted the responses of 12 cells, six in the chorda tympani nerve (lower three curves) and six in the nucleus tractus solitarius (upper three curves). Three solutions were allowed to flow over the tongue. Quinine hydrochloride, QHCl, tastes bitter at any concentration above 4 x 10-7 M. Potassium chloride is bitter at 0.03 M, but it is salty at 0.3 M concentration. The two curves for QHCl and 0.03 M KCl for the chorda tympani nerve are nearly identical as predicted (the substances taste the same), and they are noticeably different from the curve for 0.3 M KCl (that tastes different). This means that this ensemble of six neurons signals the same thing to the CNS about both bitter solutions, but it signals something different to the CNS about the salty solution. The same thing happens in the nucleus tractus solitarius. In fact, the discharges of the second-order neurons are not greatly different from those of first-order neurons except that the discharge frequencies are higher. The same is true of discharges in thalamic third-order neurons. To date, only a few cortical taste neurons have been recorded from, so their response characteristics remain a mystery. This is not for lack of attempts to record taste-related activity in the cortex. It appears that the cortex may not play a major role in gustatory sensation. <
| Smell |
The identification of primary olfactory submodalities has been attempted many times. The first serious effort at identification was made by Linnaeus, the Swedish botanist, in 1756. Over the years a large number of other classifications of odors have been made, but there is not a general agreement about primary odors in the sense of the primary tastes: sweet, sour, salty, and bitter. The human can distinguish the odors of a vast number of different molecules and describes them as aromatic, fragrant, repulsive, ethereal, resinous, spicy, burned, putrid, and so forth. Whether any of these can be considered primary is a point of debate.
Odorous substances have in common that they are either gases or volatile liquids. This is the form in which the odorant reaches the sensory epithelium, either through the nostrils with inspired air or by the back door through the mouth and throat. The receptor structures for olfaction are covered with mucus so that aqueous solubility is an asset to an odorant.
Of all the chemical elements, only 16 seem to play any role in the production of odor sensation. These are hydrogen, carbon, silicon, nitrogen, phosphorus, arsenic, antimony, bismuth, oxygen, sulfur, selenium, tellurium, fluorine, chlorine, bromine, and iodine. Only the halogens and ozone, O3, are odorous as elements. There have been many attempts to group odorants in terms of chemical structure, with some recent success. The gross profile of the molecule, i.e., its size and shape, seem to be important. Many people have also emphasized the importance of other physicochemical features, especially the characteristics of functional groups.
The olfactory system is extremely sensitive. For example, ethyl mercaptan can be detected at a concentration of 4 x 10-8 mg per liter of air. However, even at this low concentration, there would be millions of molecules in a single sniff. On the other hand, for certain odorants, a single molecule may be sufficient to excite a single olfactory receptor cell, at least in some animals. Sensitivity is no more constant in the olfactory system than in any other sensory system. It diminishes upon continuous exposure to an odor. This kind of adaptation is familiar to everyone. The smell of fried fish is not even noticed by the cook unless he leaves the kitchen and returns after some time. Odors also interact in rather specific ways. For example, adaptation to the odor of camphor results in a simultaneous increase in the threshold concentration of eucalyptus (eucalyptol) and cloves (eugenol) but not to bitter almonds (benzaldehyde). When two odorants are presented together, they can blend (violets and H2S together yield a blend smell); they can be sensed successively; one odor can mask the other (especially if one is much stronger); or the combination can give no odor at all. The perfume industry is based on these principles.
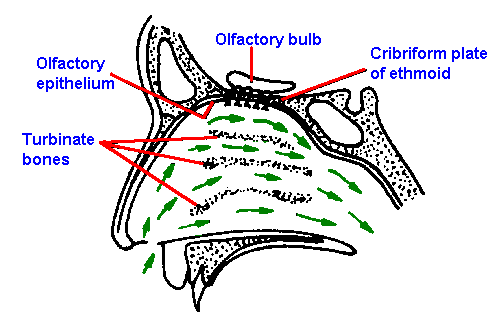 |
Figure 10-8. Sagittal section of the human nasal passages, showing the nasal fossae, the location of the olfactory epithelium and olfactory bulb, and the direction of travel of inspired air (small arrows). (Douek E: The Sense of Smell and Its Abnormalities. Edinburgh, Livingstone, 1974) |
Smell anatomy
The olfactory receptors are located in the posterior portion of the nasal cavity in a mucosal area that measures about 5 cm2. The location is shown in Figure 10-8, which is a sagittal section of the human nasal passages. The sensory epithelium sits at the top of the nasal cavity above the superior turbinate bones. It is yellow in color and has no synchronously beating cilia and therefore is distinguishable from the surrounding respiratory epithelia. Within this sensory region are found Bowman's glands that contain most of the yellow pigment and secrete much of the mucus that covers the sensory epithelium in a 10- to 40-m layer. The odorant must dissolve in this mucus layer in order to reach the olfactory receptors.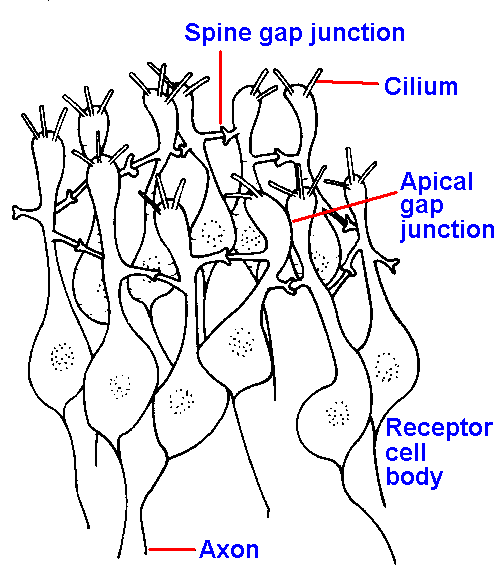 |
Figure 10-9. The olfactory mucosa showing arrangement of receptor cells in interconnected rings, one of which is illustrated. Receptor cells are connected together by gap junctions both between spines and less frequently at their apical expansions (two shown) The centers of the rings are apparently filled with supporting cells. (Graziadei PPC: Z Zellforsch 118:449-466, 1971) |
At its basal end, each receptor cell gives rise to an axon that forms a part of the olfactory nerve. Olfactory nerve fibers are unmyelinated and average about 0.2 m in diameter. In the rabbit, the olfactory epithelium contains about 100 million receptor cells, and thus there are about the same number of fibers in the olfactory nerve. In man, the olfactory nerve is short, merely perforating the cribriform plate into the brain cavity and terminating in the olfactory bulb. Olfactory nerve fibers converge onto mitral cells in the bulb, which send their axons (about 100,000 of them) in the olfactory tract to the piriform cortex, the periamygdaloid area, and the olfactory tubercle. The olfactory bulb contains interneurons, the granule cells that mediate a form of lateral inhibition between mitral cells; an active mitral cell inhibits (reduces) activity in its neighbors. There are also centrifugal fibers to the olfactory bulb that primarily mediate inhibition of mitral cell activity. The functions of this connection and of the lateral inhibition are not known.
Unlike other sensory pathways to the cerebral cortex, the olfactory pathway does not relay in the thalamus as we have just seen. However, fibers do leave the olfactory cortical areas and relay in the thalamus on their way to the hypothalamus or other areas where they perhaps play a role in the regulation of the intake of food and other behaviors that depend upon olfactory information.
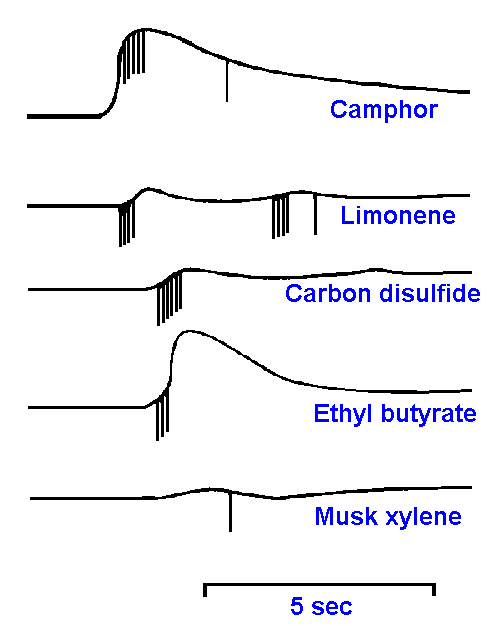 |
Figure 10-10. Responses of a single cell in the olfactory epithelium to stimulation with camphor, limonene, carbon disulfide, ethyl butyrate, and musk xylene. (Gesteland RC, Lettvin JY, Pitts WH et al.: Odor specificities of frog's olfactory receptors. In Zotterman Y [ed]: Olfaction and Taste. New York, Macmillan, 1963) |
Smell physiology
The coding mechanism for olfactory quality (submodality) has been just as elusive as the identification of the qualities themselves. Microelectrode recordings from the olfactory epithelium yield two sorts of responses to olfactory stimulation. Both can be seen in Figure 10-10. First, there is a slow-wave potential reminiscent of the generator potentials of mechanoreceptors (see Chapter 4). However, this potential is much larger than generator potentials recorded extracellularly from single receptors elsewhere. It also does not have a fixed relationship to the time of spike initiation, as can be seen in the figure. This potential probably is the summation of the generator potentials of a number of nearby receptor cells and perhaps potentials generated by supporting cells.Second, there are the familiar spike discharges in the receptor cell axons or somata. It can be seen from this typical example that receptors are not highly specific in their responses to odorants. This particular cell responded to camphor, limonene, carbon disulfide, ethyl butyrate, and musk xylene, but it was insensitive to nitrobenzene, benzaldehyde, n-butanol and pyridine. In general, each olfactory receptor responds to a variety of odorants, suggesting a very complex ensemble code.
The same sort of behavior has also been seen in mitral cells of the olfactory bulb. Perhaps, like the gustatory system, the olfactory system signals smell quality by the pattern of activity in the ensemble of olfactory receptors. The larger number of odorants compared to taste stimuli makes this hypothesis difficult to test. Studies have shown that odors may be encoded in terms of the position in the epithelium of responding receptors and the relative timing of discharges across the sheet of receptors. Spatial-temporal patterning is the basis of chromatographic separation of molecules; a similar process might be used by the olfactory epithelium in odor discrimination. Compounds with similar smells have been shown to be similar in terms of these two parameters. As yet, the evidence favoring this hypothesis must be viewed as preliminary.
| Summary |
The sensations of taste are mediated by taste buds located in papillae of the tongue. There are four taste submodalities: sweet, salty, sour and bitter. The four submodalities are not completely independent, but interact in successive tastings. Many taste stimuli (except quinine, sucrose, and NaCl) give different sensations at different concentrations. Taste receptors normally respond to more than one of the taste submodalities; thus, taste quality is probably encoded as an ensemble code. Taste intensity is probably encoded in terms of the total number of impulses (or frequency) discharged in all the fibers sensitive to a particular substance. Substances that taste the same tend to excite taste receptors in a similar way. Olfactory sensations are evoked by volatile substances, but primary submodalities have not been identified. Olfactory receptors respond to a variety of odorants, and thus some kind of ensemble code is likely for this modality as well.
| Suggested Reading |
1. Amoore JE: Stereochemical theory of olfaction. In Schultz HW, Day EA, Libbey LM [ed]: Chemistry and Physiology of Flavors. Westport, CT, Avi Publishing Co, 1967.
2. Beidler LM [ed]: Handbook of Sensory Physiology. IV. Chemical Senses. 1. Olfaction. New York, Springer, 1971.
3. Beidler LM [ed]: Handbook of Sensory Physiology. IV. Chemical Senses. 2. Taste. New York, Springer, 1971.
4. Mozell MM: The spatiotemporal analysis of odorants at the level of the olfactory receptor sheet. J gen Physiol 50:25-41, 1966.
5. Shepherd GM: Synaptic organization of the mammalian olfactory bulb. Physiol Rev 52:864-917, 1972.
6. Shibuya T, Tucker D: Single unit response of the olfactory receptors in vultures. In Hayashi I [ed]: Olfaction and Taste. II. Oxford, Pergamon Press, 1967.
1. The response of a single fiber to each stimulus is found by reading up columns indicated by the letters on the abscissa.
[TOC] [Chapter 11] [Glossary] [Index] [Abbreviations]