
 Chapter 4
Chapter 4

RECEPTOR PROPERTIES: RECEPTOR POTENTIALS AND CODING
Let us begin our discussion of the neurophysiology of sensation by considering what happens in a receptor when a stimulus is applied to it. In later chapters individual sensory receptors for each sense will be considered separately, but at this point, it is the general properties of receptors that are of concern. We have already seen that when a suprathreshold stimulus (of strength greater than threshold strength) is applied to a receptor, the nerve fiber associated with that receptor discharges. What we would like to know is how the stimulus energy is transformed into action potentials in the normal operation of the system, i.e., what is the nature of the transduction process (the process of converting stimulus energy into spikes)?
| Receptor potentials |
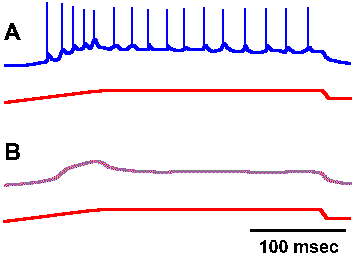 |
Fig. 4-1. Relation between impulse response and underlying generator potential. A. Response of a muscle spindle receptor to prolonged stretch, with nerve recording shown in the upper trace and monitor of the stretch of the muscle in the lower trace. B. The same response after bathing the spindle in 0.2% lidocaine (Ottoson D and Shepherd GM: Acta physiol scand 79:423-430, 1970). |
The transduction process appears to be similar in all of the various types of receptors that have been studied. As an example of a transducer, let us consider the muscle spindle, a small structure found in skeletal muscle that contains two kinds of receptors both of which can signal the length of the muscle. The anatomy and physiology of the muscle spindle will be considered in more detail in Chapter 11. If a recording electrode is placed on the sensory nerve fiber supplying one of the receptors in the muscle spindle (the primary receptor) at a point very near the spindle itself and then the spindle is stretched, recordings like those in Figure 4-1 result. The upper trace of each pair shows the recording from the fiber, whereas the lower trace shows the monitor of the muscle length (longer being indicated by an upward deflection). In Figure 4-1A, the spike discharge is superimposed upon a slow hypopolarizing potential that develops as the muscle is stretched. If the preparation is bathed in a solution of lidocaine, a local anesthetic agent, or in tetrodotoxin, the action potentials are blocked as in Figure 4-1B, leaving the slow hypopolarizing potential by itself. This potential, called the generator potential, can be shown to originate in part of the spindle receptor, not in the nerve fiber. There are receptors that are actually nerve fibers, part of which has been specialized to be sensitive to stimuli (e.g., receptors in the olfactory bulb and free nerve endings in skin), and there are receptors that are other types of cells in close association with nerve fibers (e.g., Merkel's disks in skin and sensory cells in taste buds). In the latter case, there appears to be a synaptic connection between the nonnerve cell and the nerve cell (the receptor), and the nerve fibers are usually not very sensitive to the same stimulus that excites their receptors.
Some people use the term generator potential synonymously with receptor potential, whereas others prefer to reserve the term receptor potential for those cases where the receptor does not itself generate spikes, as in the receptors of the eye. We will use the term receptor potential here in this latter sense.
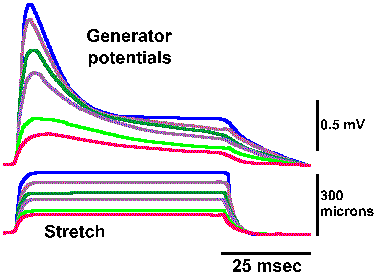 |
Fig. 4-2. Graded responses of a muscle spindle receptor to stretch. Graded stretches are indicated by the stretch monitor in the lower traces; graded generator potentials are shown in the upper traces (Ottoson D and Shepherd GM: Cold Spring Harbor Symp Quant Biol 30:105-114, 1965). |
The generator and receptor potentials are local or non-propagated events as shown by the degradation of their amplitudes with increasing distance from the receptor. One need only move the recording electrode along the nerve a few millimeters farther away from the position near the spindle that produced the records in Figure 4-1 before the generator potentials disappear. They are graded responses as opposed to the all-or-none character of the action potential; the amplitude of the generator and receptor potentials increase with increasing stimulus strength. Different amounts of muscle stretch, as shown by the heights of the indicators superimposed in the lower trace, resulted in the graded series of generator potentials superimposed in the upper trace of Figure 4-2. For these records, the action potentials have been blocked by application of lidocaine. The graded increases in the generator potential amplitude with graded stretches of the muscle are clearly illustrated in this figure.
| The channels opened during the generator potential are non-specific; they admit all small ions. |
The steps in formation of a generator potential are not known for every receptor, but where it has been studied the start of the generator potential usually results from an increase in the permeability of the membrane of the receptor to all small ions, but the ion furthest from its electrochemical equilibrium and in greatest concentration, namely sodium, contributes the greatest current. As during an action potential, an increase in sodium conductance leads to an hypopolarization. The ionic mechanism of the generator potential is similar to that for the action potential but with a longer time constant; however, the restoration of the membrane potential to resting values at the end of the generator potential is a passive process not involving increased potassium conductance as in the action potential.
The transduction process takes place within the receptor region of the cell, that region that is sensitive to adequate stimuli and is responsible for the generation of the generator potential. The membrane of the receptor region is, however, electrically inexcitable; it contains no voltage-gated ionic channels and does not generate spikes. If the receptor region generated action potentials, the graded nature of the generator potential would be destroyed because as soon as the generator potential exceeded the critical firing level an action potential would be initiated, reversing the membrane polarization no matter how large or small the stimulus, i.e., the membrane potential would no longer encode the stimulus intensity. The action potentials themselves are usually initiated in a physically separate region of the cell, the pacemaker or spike-generating region. This may be anatomically separate from or continuous with the receptor region or with the axon itself, the conducting region. For receptors that do not themselves generate spikes, the spike-generating regionis in a different cell. For example, receptor potentials occur in the rods and cones of the eye, but the first spikes in the visual system occur in the ganglion cells. Figure 4-3 shows how a receptor can conceptually be divided into a receptor region, a spike-generating region and a conducting region. As illustrated in the figure, the inward (sodium) current that hypopolarized the active (i.e., with changed conductances) receptor region is concomitant with an outward current (possibly carried by potassium ions) in the spike-generating region of the cell. That this must be true follows from Kirchhoff's current law; current flows only in complete circuits. As anywhere in a nerve cell, outward current though an inactive (i.e., with normal resting conductances) region of membrane causes an hypopolarization of that membrane. An hypopolarization, as we already know, leads to the formation of action potentials, provided that the critical firing level is reached or exceeded. Actually, the spike-generating region simply responds to hypopolarizing current in the same way as any other membrane containing voltage-gated Na+ and K+ channels. The conducting region of the membrane of the sensory receptor simply "follows" what the spike-generating region does; it simply transmits, without alteration, signals it receives.
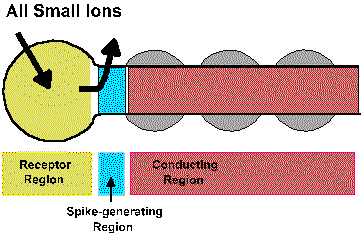 |
Fig. 4-3. A schemtatic diagram of a generaized receptor showing the receptor region, the spike generating region, and the conducting region along with the currents that flow during the generator potential. |
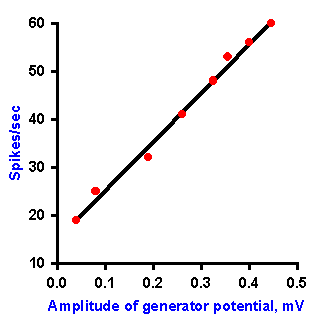 |
Fig. 4-4. Relation between amplitude of the generator potential and frequency of impulse discharge during the dynamic phase of stetch [time when muscle and spindle are actually changing length] (Ottoson D and Shepherd GM: Acta physiol scand 79:423-430, 1970). |
In the absence of an anesthetic agent, it is possible to study the relationship between the frequency of discharge in the spindle axon and the amplitude of the generator potential during the dynamic phase of stretch (i.e., the time when the muscle and the muscle spindle are actually changing length). The relationship obtained for various muscle lengths is linear, as plotted in Figure 4-4. Again, recall that the fact that the plot is linear indicates that equal increments in discharge frequency result from equal increments in generator potential amplitude. A linear relationship between the amplitude of the generator potential and the discharge frequency of the nerve has been found for every receptor studied so far. For the muscle spindle, there is also a linear relationship between the amount of stretch applied and the amplitude of the generator potential. The muscle spindle is one of the cases of a receptor in which there is a linear relationship (the exponent of the power function, k=1) between the strength of the stimulus and the response of the cell, i.e., the amount of stretch and the frequency of discharge of the associated nerve fiber. If the relationship between stimulus and response is curvilinear for a sensory receptor, as in those cases where the relationship is a logarithmic or power function (the exponent, k1), then there is a curvilinear relationship between the strength of the stimulus and the amplitude of the generator potential, and, in fact, it is a logarithmic or power function. This must be the case if the relationship between the amplitude of the generator potential and the discharge frequency is always linear, because a linear transformation of any function or relationship always gives the same sort of function or relationship. It is important to bear in mind that the normal sequence of events in transduction is
- stimulus
- receptor or generator potential
- action potentials
| The relationship between generator potential amplitude and frequency of discharge is always linear. |
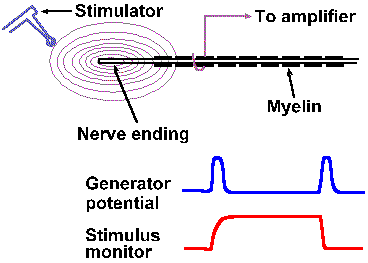 |
Fig. 4-5. A. The setup for recording generator potentials from Pacinian corpusles. B. The stimulus monitor and an example of a Pacinian generator potential. |
Summation
In receptors that are suitable for applying two stimuli close together, two other properties of generator potentials can be studied. If a mechanical stimulus is applied to a mechanoreceptor, a generator potential is recorded such that the amplitude of the generator potential is a function of the amount of deformation of the receptor surface. Two identical stimuli applied in rapid succession at the same site result in a generator potential twice the size of that resulting from a single stimulus. In fact, the amplitude of the generator potential that results from any two stimuli applied in close temporal proximity is always equal to the algebraic sum of the generator potentials initiated by each stimulus alone. An example of this behavior is shown in Figures 4-5 and 4-6 for a Pacinian corpuscle, a tiny ellipsoidal body composed of a number of concentric lamellae and with a myelinated fiber running into its center. The corpuscles are found in connective tissue of the mesentery and the popliteal fossa among other places; their function is largely unknown but they seem suited to sense and signal vibration. Their generator potentials have been thoroughly studied. Figure 4-5 shows the way a mechanical stimulator is applied to the isolated corpuscle while recordings are being made from its axon. When the stimulator presses down on the corpuscle, as shown in the lower trace by an upward deflection of the stimulus monitor trace, an hypopolarizing generator potential is initiated in the receptor (upper trace). The potential rapidly returns to resting levels, but a new hypopolarizing generator potential is set up when the corpuscle is released from compression. This behavior is typical of the Pacinian corpuscle. If we were recording from the nerve fiber associated with the corpuscle at a site farther away, we would record an action potential associated with each of the generator potentials, but for now we are concerned about the generator potentials. We can use the first generator potential of the corpuscle to study summation. As shown in Figure 4-6, stimulus A results in a generator potential of amplitude a and stimulus B (applied slightly later in time than A) in a potential of amplitude b. When A and B are applied together, the resulting generator potential has amplitude a + b. This additive property of generator potentials is called temporal summation, because the variable is time. A similar phenomenon results when two stimuli are applied at the same time at two different locations. Again in Figure 4-6, stimulus C results in a generator potential of amplitude c, whereas stimulus A+C results in a generator potential of amplitude a + c. In this case, the variable is the spatial distribution of the stimuli instead of time; thus the phenomenon is termed spatial summation. Figure 4-6 shows summation of only two generator potentials, but any number of generator potentials can summate in a single cell, limited only by the physical arrangement of the receptor, i.e., how many stimuli can impinge upon it at the same time.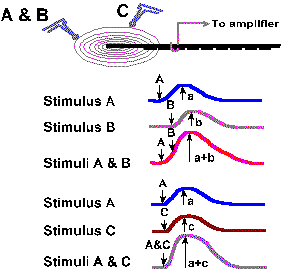 |
Fig. 4-6a. Spatial and temporal summation of generator potentials in a Pacinian corpuscle. Stimuli A and B are applied, with the same stimulator, close together in time. Stimulus C is applied at another place on the receptor at the same time as A in the second set of records. |
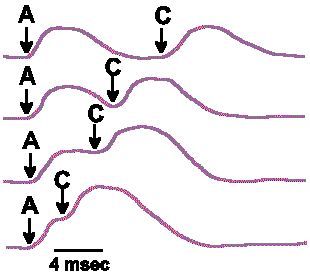 |
Fig. 4-6b. The summation that results from varying the interval between stimuli A and C as delivered in Fig. 4-6a. |
The generator potential rises from the resting membrane potential, reaches a peak after a given interval, then returns toward the resting membrane potential. If the stimuli are presented so far apart in time that the first generator potential is over before the second stimulus is applied, then there will be no summation. Thus, the amplitude of the summated generator potential depends upon how much the membrane potential of the receptor is altered from the resting potential at the moment the second stimulus is applied. At the bottom of Figure 4-6 are shown a number of examples of how summation is affected by varying the time between the two stimuli, A and C; the maximum summed potential occurs when the stimuli are delivered simultaneously and the amplitude falls off as the second stimulus is delayed. The end result of the summation is an increase in the frequency of discharge of the sensory nerve fiber over the frequency that would be obtained for either stimulus alone. Any number of generator potentials can sum in this same manner, each increment in the amplitude resulting in increased discharge frequency, until a maximum frequency, characteristic of that particular fiber, is reached.
Adaptation
The time over which summation can be obtained depends upon the duration of the individual generator potentials. To a certain extent, the duration of the generator potential depends upon the duration of the stimulus; however, some receptors have generator potentials that last only a short time, no matter how long the stimulus is maintained. Figure 4-7 shows the generator potentials of a Pacinian corpuscle and a muscle spindle obtained for a sustained deformation and stretch, respectively. Notice that the generator potential for the corpuscle increases to a maximum amplitude and then returns to the resting membrane potential despite the persistence of the stimulus. On the other hand, the muscle spindle generator potential increases to a maximum and then declines a bit, but is maintained above the resting potential for the duration of the stimulus. We refer to a decrease in the amplitude of the generator potential or the frequency of discharge of the sensory fiber in the face of a persisting, constant stimulus as adaptation. Receptors that behave like Pacinian corpuscles are said to be rapidly adapting; those that behave like muscle spindles are said to be slowly adapting. For some receptors, the rate of adaptation is a function of the physical characteristics of the receptor structure. In the Pacinian corpuscle, which shows an extreme of rapid adaptation, the adaptation is slowed considerably by removal of the lamellae and application of the stimulus directly to the nerve fiber. In this case, the adaptation occurs because the coupling of the stimulus to the receptor is redueced. Membrane accommodation (see Chapter 3) may also play a role in producing adaptation.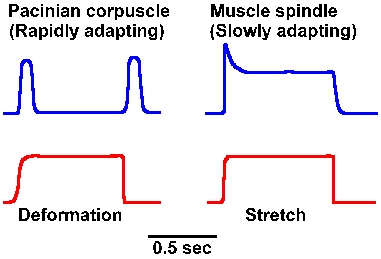 |
Fig. 4-7. Comparison between rapidly adapting (Pacinian corpuscle) and slowly adapting (muscle spindle) generator potentials. |
The reason for having both slowly and rapidly adapting receptors lies in the different kinds of information they signal. Slowly adapting receptors presumably signal the onset and offset, but most importantly the presence of a long sustained stimulus. They do adapt to some extent as indicated by the gradual waning of the sensation of the chair against your posterior as you read this. (This probably does not represent entirely a receptor process, but involves the central nervous system as well, as is indicated by the fact that the sensation returned to your posterior as soon as mention was made of its absence.) In contrast, rapidly adapting receptors signal the start, velocity, and, in some cases, the stop of a stimulus, but do not signal in between. On the other hand, they give very good responses to repeated stimuli, suggesting they may play a role in sensations of vibration. The time over which summation of slowly adapting generator potentials can occur is longer than for rapidly adapting ones for a stimulus of the same duration.
| Coding |
At one time the nervous system was thought to be a syncytium, a continuous, interconnected, multinucleated mass of protoplasm. The problem of explaining how information was routed from one place to another was most puzzling in such a system, because any stimulus should in theory be able to set the whole mass alive with impulses--all identical. How could any part of the system operate on its own; how could we tell hearing from smelling, high pitches from low? We now know that nervous systems are composed of individual cells strung together and able to communicate with each other in specific patterns. Even with this "simplification," the problem of how the information contained within a stimulus is signaled to distant parts of the nervous system is formidable.
Any stimulus contains within it certain features that are of interest to the organism. Stimuli have
- intensities or strengths
- locations or sites of application
- frequencies of application
- rates of application
- modalities
Some physiologists prefer to define modalities of sensation in terms of the stimuli that produce them. They refer to touch, pressure, and pain as modalities or submodalities of somesthesia with particular reference to the way the skin is stimulated. But, do touch, pressure, and pain represent different forms of stimulation or just different strengths of the same stimulus? With your fingers, lightly grip a fold of skin on your forearm, a stimulus you might consider as touch. A stronger grip yields a sensation of pressure, and an even stronger grip a sensation of pain. Yet the manner of stimulation is the same in all three cases. Taking another example, electrical stimulation of a neuron can lead to the same sensation as a more natural stimulation of the skin. Clearly, electrical energy is not the same form as mechanical energy, still the quality of the sensation is the same.
In our discussion, we use the term modality to mean one of the following:
- vision
- audition
- gustation
- olfaction
- somesthesia (including skin, muscle, position, and visceral senses)
How do neurons recognize and encode the features of a stimulus? The term coding means simply the representation of the facts about the stimulus (information) in terms of neural activity. Most neurons generate action potentials by which they communicate with each other. These action potentials are essentially the same for all neurons. For the neurophysiologist studying coding, the problem is rather like that of a nation that intercepts a secret code sent by a foreign nation--what does the message say? There are quite a few codes possible using neural activity, many or all of which are used somewhere in the nervous system. For the present, the discussion will concentrate on five classes of neural codes, with some specific examples of how they may be used.
Code of specific nerve energies
Typically, sensory receptors are sensitive to many different kinds of stimulus energy. For instance, receptors in the hand respond to touch, heat, or vibration of the skin; the nerve itself can also respond to mechanical stimulation. Anyone who has ever hit his "funny bone" can attest to this fact. However, every receptor has one form of stimulus energy to which it is most sensitive, and this stimulus form is called the adequate stimulus. The eye is excitable most easily by light, although it can also be excited by pressure on the eyeball itself; therefore, for the eye, light is the adequate stimulus. The neurophysiological term adequate stimulus, does not refer to stimulus strength, only to a form of energy. It is quite possible to have a subthreshold adequate stimulus.
| Careful: it is possible to have a subthreshold adequate stimulus. |
Complementary to the principle of the adequate stimulus is a notion formulated by Johannes Müller. Müller noticed that, though the eye is excitable by light, pressure on the eyeball, electrical stimulation of the optic nerve, and some irritative pathological conditions, the sensation experienced is always one of vision--the person "sees light." The Doctrine of Specific Nerve Energies, as formulated by Müller, says that, although a sense organ may be sensitive to many forms of stimulus energy other than its adequate stimulus, the sensation evoked is always like that associated with the adequate stimulus, no matter what kind of energy was applied. With electrical stimulation of the optic nerve, the sensation evoked is one of seeing light, not one of an electrical shock. The doctrine of specific nerve energies implies that the modality or submodality of a sensation is determined not by the stimulus, but by what specific receptor or nerve fiber is stimulated. The doctrine also implies that the subjective qualities of a modality are determined, not in the receptors themselves, but in the central nervous system.
An extension of the doctrine of specific nerve energies is the concept of labeled lines . The concept of labeled lines says that information from a particular receptor travels over particular pathways to particular parts of the nervous system. Thus, the modality of a sensation depends upon which particular cell, pathway, nucleus or lobe is activated by the stimulus. The concept of labeled lines receives some support from the fact that stimulation of the spinothalamic tract in man causes pain or from the observation that stimulation of the visual cortex leads only to a perception of light in a place within the visual world that depends upon where the visual cortex is stimulated.
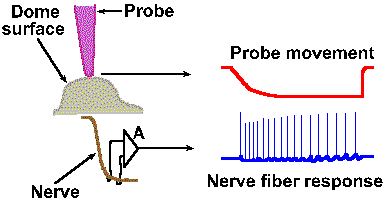 |
Fig. 4-8a. An example of a frequency code. The physical setup of the experiment illustrates how the probe is positioned over the receptor structure and how the discharges of the nerve fiber are recorded. At the right, typical response from the fiber (lower trace) to prolonged displacement (upper trace) of the receptor surface (downward deflection of the trace indicates a downward movement of the probe). Entire record is 40 msec in duration. |
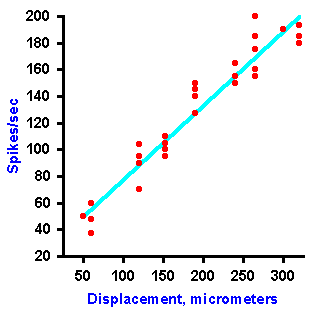 |
Fig. 4-8b. A plot of the frequency of discharge of the fiber against the amount of displacement of the receptor. Notice that the amount of displacement is coded as a linear function of the frequency of discharge (Data from Tapper DN: Trans NY Acad Sci 26:697-701, 1964). |
The concept of labeled lines implies that between the receptor on the periphery and the place in the central nervous system where the sensation occurs, there is no interaction between the modalities or submodalities. This does not seem to be true. For example, in the cuneate nucleus, a major relay in the somatosensory system, there is interaction between activity arising from hair receptors and activity arising from touch receptors. In addition, there is interaction here between cutaneous, auditory, and visual activity. The nature of this interaction is just beginning to be explored. It is true, however, that some structures within the central nervous system do appear to be associated with particular modalities of sensation.
Even if a specific pathway or labeled line underlies the subjective quality of a sensation, a given neural pathway must use more than one code if other stimulus features are to be signaled. Whereas any activity in the pathway would evoke an experience of a specific quality or modality, the intensity of the sensation is usually coded within the activity itself.
Intensity can be coded in terms of discharge rate. Many mechanoreceptors use modulation of their discharge rates, that is, the number of impulses generated per second, to code the intensity of a mechanical stimulus. Figure 4-8 illustrates a rate code for the amount of displacement of the surface of a Merkel's disk mechanoreceptor found in the hairy skin of cats and on the abdomens of humans. In this experiment, a probe was applied to the dome-shaped surface of the receptor, as shown in A. The probe was pushed down, indenting the surface a certain distance, and held there while recordings were made from the sensory nerve fiber supplying the receptor. A typical 40-msec recording is shown in B. The upper trace illustrates the movement of the probe (a downward deflection of the trace indicates a downward movement of the probe), whereas the lower trace is an actual recording of the fiber's discharge. The average frequency of discharge, measured during the time when the displacement was held constant (the plateau), for a number of different displacement distances are plotted in the graph of Figure 4-8C. Notice that the fiber codes increasing displacement by increasing its discharge rate in a linear fashion. The equation defining this relationship is F = 0.55D + 18, where F is the rate of discharge in spikes/sec and D is the displacement in micrometers. Knowing this relationship, we can derive the amount of displacement the receptor must have experienced from the frequency of discharge of its nerve fiber. Presumably the nervous system can do this, too. Most neurons generate impulses or action potentials, but they spend most of their time in the resting condition. Even a neuron that is generating impulses at a rate of 50/sec spends most of its time resting. This can be seen in Figure 4-9. If an action potential lasts 0.5 msec, as shown in A (some action potentials are shorter and some are longer), and repeats at a rate of 50/sec, as shown in B, the neuron is silent for 97.5% of the time. Interestingly, one of the possible codes of neural activity is based on this silent time.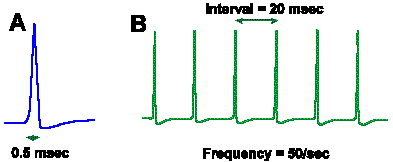 |
Fig. 4-9. A. The duration of a single nerve impulse. B. The interval between impulses in a train of nerve impulses at 50/sec. |
For vibrating stimuli, it is possible to encode the frequency of the stimulus in a linear fashion with one impulse for each stimulus, as a frequency code, or with one impulse for every two or more vibrations of the stimulus, as an interval code. Coding of vibratory frequency is perhaps the most trivial example of a possible interval code and, at the same time, the least different from a frequency code. When the stimulus is not periodic, the interval code is more obvious. It is possible that in some cells, such as pyramidal tract cells, a particular short interval between action potentials, in a train where the other intervals are much longer, may signal (code) the start of some movements. We will examine this in more detail later. Briefly, however, this phenomenon is related to a special event called facilitation that occurs in certain postsynaptic cells only when two presynaptic action potentials arrive at short intervals.
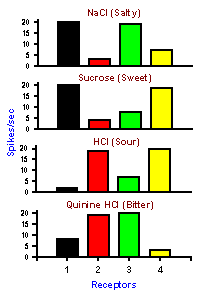 |
Fig. 4-10. An example of a pattern code. Four different taste receptors: Each responds to each of 4 substances of different tastes. None by itself signals the substance applied, but by looking at the pattern of discharge in all 4 receptors it is possible to deduce what the substance was. |
Neuron pattern or ensemble codes
In some cases, one neuron may not be capable of signaling all the features of a stimulus that we can sense. In these cases, the stimulus features may be signaled by activity in populations of neurons and decoded by comparison, addition or subtraction of their activity. It is the ensemble of cells that carries the information about the particular sensation. An example of a possible pattern or ensemble code is the coding of the submodalities of taste: sweet, sour, salty, and bitter. Every gustatory receptor responds to substances of all four qualities. The responses of four such receptors are shown in Figure 4-10.Each column of the figure represents the responses of a single taste receptor to four different solutions, one from each submodality. The height of the bar indicates the magnitude of the response that particular receptor gave when the particular solution was poured over it. Looking at the response of only one receptor, it is impossible to tell what the stimulus was. For example, receptor 1 responds equally to salty and sweet and thus cannot distinguish between the two. By comparing the responses of the four cells, it is possible to detect which stimulus was presented. Salty corresponds to large discharges from receptors 1 and 3, sweet to large discharges from receptors 1 and 4, and so on. There is a unique pattern of discharge across the group of receptors for each different quality, even though no one receptor can signal a given quality uniquely; obviously, there are more than four taste receptors in the tongue, but a similar principle could be applied to a much larger set of receptors.
Nonimpulse codes
To this point, we have considered codes involving the generation of propagated action potentials. Why do nervous systems use action potentials to communicate information? Wouldn't it be easier to communicate increasing stimulus intensity with increasing amplitude of a voltage? The answer to both of these questions is quite simple. Voltages are attenuated drastically in a very short distance in volume conductors. Where long distances must be traversed it is imperative to use a brief, self-regenerating potential change that stays the same size all along the fiber. Coding can then be accomplished by modulating the frequency of discharge.However, some cells in the nervous system do not generate action potentials at all. In these cases, the distances traversed are only micrometers, and reduction of potential with distance is no problem. For example, in the eye, information must pass through a minimum of two cells before any action potentials are generated. All of the information in the optic nerve discharges must also be encoded in the hypopolarizations and hyperpolarizations of the receptor cells, horizontal and amacrine cells, and bipolar cells.
Any time the potential across the membrane of a cell changes there is a current flow generated in the extracellular space. These currents are large enough in the cerebral cortex to be recordable on the scalp as the electroencephalogram or EEG. Small amounts of these extracellular currents can penetrate other nearby cells, altering their membrane potentials and thus their excitabilities. This kind of interaction can sometimes be quite powerful. We presume that this is another manner in which information can be coded and transferred, but as yet there is no proof that this is so.
| Summary |
Transduction is a four-stage process. The stimulus causes:
- a local change in membrane permeability, which in turn allows
- the generator current to flow, leading to
- a local hypopolarization, the generator potential.
- finally, the generator potential gives rise to the propagated spike in the same or an adjacent region of the membrane
The generator potential is not conducted, but invades the region of spike generation electrotonically. Generator potentials can be summed both temporally and spatially. Receptors differ in their rates of adaptation, depending upon physical properties of the receptor structure and other factors, including accommodation.
There are a number of possible ways that information can be coded in the nervous system. It can be coded in terms of which elements are active--the "doctrine of specific-nerve-energies"--and the labeled line notion. Information can be coded in terms of variations in impulse trains, either their frequencies or the patterns (intervals) of the impulses. It can also be coded as variations in the amount of activity in different elements of an ensemble of neurons. Finally, it can be coded in terms of non-propagated or electrotonic potential changes, as long as transmission distances are very short.
| Suggested Reading |
- Aidley DJ: The Physiology of Excitable Cells. Cambridge, Cambridge University Press, 1971.
- Loewenstein WR [ed]: Principles of Receptor Physiology. Handbook of Sensory Physiology, Vol. I. Berlin, Springer, 1971.
- Mountcastle VB: Sensory receptors and neural coding: introduction to sensory processes. In Mountcastle VB [ed]: Medical Physiology, 13th ed, Vol. 1. St. Louis, Mosby, 1974.
- Rushton WAH: Peripheral coding in the nervous system. In Rosenblith WA [ed]: Sensory Communications. Cambridge MA, MIT Press, 1961.
- Zimmermann, M: Neurophysiology of sensory systems. In Schmidt RF [ed]: Fundamentals of Sensory Physiology. New York, Springer, 1978.
[TOC] [Chapter 4b] [Glossary] [Index] [Abbreviations]